Chapter 13 Study Guide
advertisement
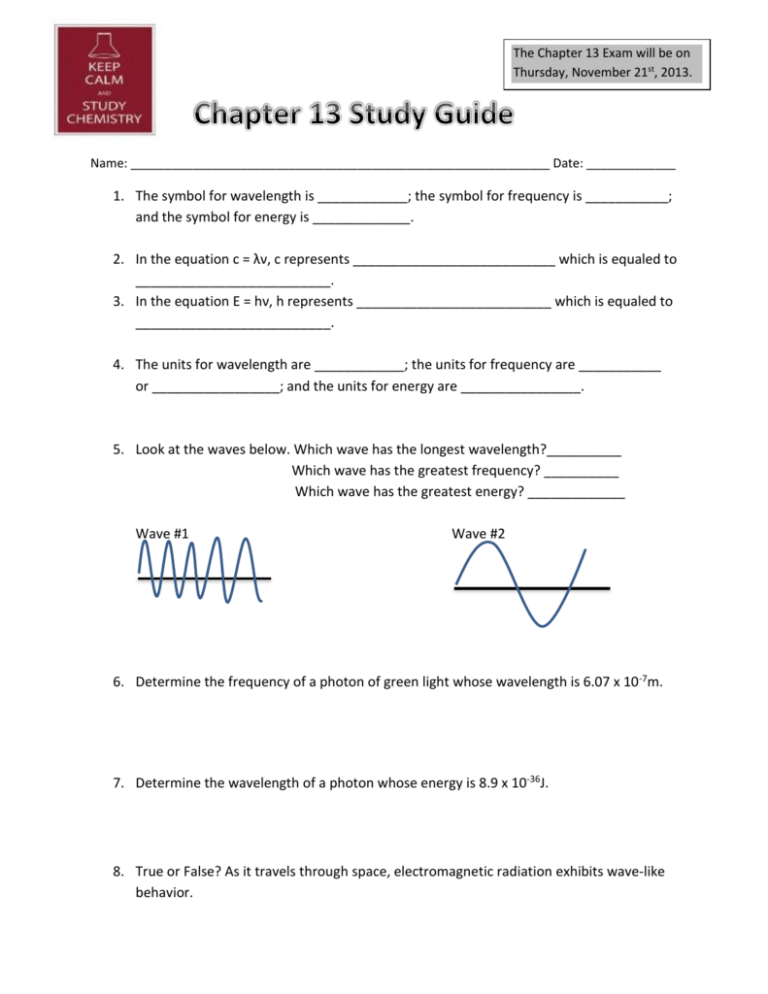
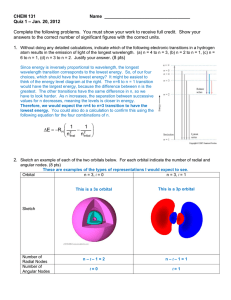
The Chapter 13 Exam will be on Thursday, November 21st, 2013. Name: _____________________________________________________________ Date: _____________ 1. The symbol for wavelength is ____________; the symbol for frequency is ___________; and the symbol for energy is _____________. 2. In the equation c = λν, c represents ___________________________ which is equaled to __________________________. 3. In the equation E = hν, h represents __________________________ which is equaled to __________________________. 4. The units for wavelength are ____________; the units for frequency are ___________ or _________________; and the units for energy are ________________. 5. Look at the waves below. Which wave has the longest wavelength?__________ Which wave has the greatest frequency? __________ Which wave has the greatest energy? _____________ Wave #1 Wave #2 6. Determine the frequency of a photon of green light whose wavelength is 6.07 x 10 -7m. 7. Determine the wavelength of a photon whose energy is 8.9 x 10-36J. 8. True or False? As it travels through space, electromagnetic radiation exhibits wave-like behavior. 9. A three dimensional region around the nucleus sometimes referred to as “the zone of probability” is called a(n) _________________. 10. If electrons in an atom are in the lowest possible energy state, the atom is said to be in the _____________________ state. 11. When a metal salt of sodium is heated, a bright orange flame is produced. Describe this phenomenon with regard to the electrons. ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 12. The set of orbitals that are dumbbell shaped and directed along the x, y, and z axes are called ___________________________. 13. A spherical electron orbital is a(n) ___________ orbital. 14. The specific name for the f orbital is a(n) _____________________. 15. How many electrons can occupy the s orbital? _______________ They must also have __________ spins. 16. What is the electron configuration for nitrogen? ___________________________ 17. What is the electron configuration for palladium? _______________________________________________________________________ 18. What is the noble gas configuration for Yttrium? __________________________________ 19. What is the noble gas configuration for Sulfur? ____________________________________ 20. Using arrows to represent electrons, write the electron notation for Fluorine. 21. Using arrows to represent electrons, write the electron notation for Magnesium.