Scientific Notation and Significant Figures

Name: ___________________________________________________________________ Date: __________________________ Pd: __________
Accelerated Physics
Mr. Roberts
Scientific Notation and Significant Figures
I. Scientific Notation & Significant Digits
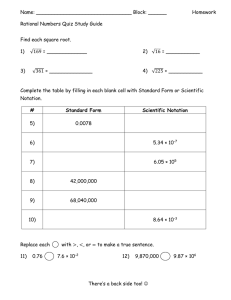
A. Express each of the following numbers in scientific notation and the correct number of significant digits
1. 2370 m _________________________________ __________
2. 0.3 g
3. 140.02 m/s
_________________________________
_________________________________
__________
__________
4. 0.000 045 amp
5. 0.000 00707 mL
_________________________________
_________________________________
__________
__________
B. Express each of the following in ordinary notation and list the correct number of significant digits
1. 5.63 x 10 -3 m 2 _________________________________ __________
2. 6.7 x 10 5 cm
3. 1.01 x 10 3 mg
_________________________________
_________________________________
__________
__________
4. 2.002 x 10 -4 kg _________________________________ __________
C. Perform the indicated operations. Place all answers in proper scientific notation and list the number of significant digits. Include units where appropriate.
1. (2.60 x 10 -3 m)(1.2 x 10 4 m)
2. (8.7 x 10
3. 1.01 x10
9.04 x 10
5
3
2
cm)(2.4 x 10
mg mL
4. 2.002 x 10 -4 kg
3.00 x 10 -8 m/s 2
-3 cm)
_________________________________
_________________________________
_________________________________
_________________________________
________
________
________
________
5. 2.00 x 10 -3 cm + 3.02 x 10 -2 cm _________________________________
6. 3.56 x 10 2 s - 4.5 x 10 -3 s _________________________________
________
________
7. 1.0009 x 10 4 m/s - 3.3 x 10 3 m/s __________________________________ ________
8. 2.2 x 10 3 N + 1.02 x 10 3 N __________________________________ ________
II. Dimensional Analysis: Solve the following problems using dimensional analysis only. Include units and show all work.
1. How many kiloseconds are in 495 seconds?
2. How many meters in 0.007 mm?
3. Convert 70 mph to km/h.
4. Convert 3.00 x 10 8 m/s (the speed of light) to miles per hour.
5. Convert 2.03 m/s 2 to miles per hour/second.
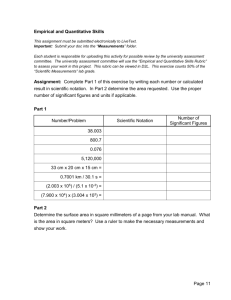
III. Algebraic Conversions: Solve for the algebraic quantity in bold.
1. F = ma, a = 2. o F = 1.8
o C + 32, o C =
3. V = lwh, h =
5. v f2
= v i2
+ 2ad, d = 6. d = v i
4. v t f
= v i
+ 1/2gt
+ at, a =
2 g =
IV. Graphing On the separate sheet of graph paper, plot the data in the following table.
Make sure to label the axes.
Motorized Cart moving along a table.
Time
(seconds)
0.0
0.5
1.0
Position
(cm)
0.0
25.0
49.0
1.5
2.0
2.5
3.0
75.5
101.0
125.0
151.5
3.5 175.5
1. Calculate the slope of the line. _________________________
2. What are the units of the slope of the line? ________________________
3. Write the equation of the line. ______________________________
Plot the data table here. Label your axes