pubdoc_2_22230_1577
advertisement

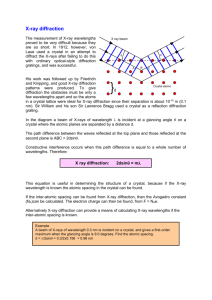
X-ray Diffraction Introduction: When x-rays were discovered in 1895 by Wilhelm Conrad Röntgen (the first Nobel laureate in Physics1901), their exact nature was not known. The question posed at the time was "are x-rays particles or are they waves like visible light?" Showing that xrays could diffract would prove that x-rays have a wave-like nature. If x-rays were waves, then their wavelengths had already been estimated to be roughly10-9 cm. Diffraction effects are observed only when the repeat distances in a material are of the order of magnitude of the wavelength of the radiation. At that time it was strongly believed that crystals were made up of many repeating blocks and that each repeating block contained a constant number of the same type of atoms. From some simple calculations using density, atomic numbers, and Avogadro's number, researchers of the time were able to show that simple crystals should have repeating units of the size needed for their proof of the wave-like nature of x-rays. Friedrich and Knipping performed the first x-ray diffraction experiment using a crystal of copper sulfate.( W. Freidrich, P. Knipping, and M. Von Laue, Proc. Bavarian Acad. Sci., 1912, 303;Reprinted in Naturewissenschaften, 1952, 39, 367.) They obtained a diffraction pattern and concluded that x-ray must be electromagnetic radiation. Bragg diffraction was first proposed by William Lawrence Bragg in 1912 as a means of analyzing the structure of crystals. Bragg and his father William Henry Bragg collimated x-rays to diffract off of different crystal planes. The x-rays were then collected in an ionization chamber and the level of ionization was measured as a function of the incident angle of the x-rays. Using this method, the Braggs were able to determine the crystalline spacing for a number of substances. In 1919 A. W. Hull gave a paper titled, “A New Method of Chemical Analysis.” Here he pointed out that “….every crystalline substance gives a pattern; the same substance always gives the same pattern; and in a mixture of substances each produces its pattern independently of the others.” X-ray Production: 1-When high energy electrons strike an anode in a sealed vacuum, x-rays are generated. Anodes are often made of copper, iron or molybdenum. 2- X-rays are electromagnetic radiation. 3- They have enough energy to cause ionization. Theoretical Considerations In order to better convey an understanding of the fundamental principles for x-ray diffraction instruments, let us quickly look at the theory behind these systems. Solid matter can be described as: Amorphous: The atoms are arranged in a random way similar to the disorder we find in a liquid. Glasses are amorphous materials. Crystalline: The atoms are arranged in a regular pattern, and there is as smallest volume element that by repetition in three dimensions describes the crystal. E.g. we can describe a brick wall by the shape and orientation of a single brick. This smallest volume element is called a unit cell. The dimensions of the unit cell are described by three axes: a, b, c and the angles between them alpha, beta, gamma. About 95% of all solids can be described as crystalline. An electron in an alternating electromagnetic field will oscillate with the same frequency as the field. When an x-ray beam hits an atom, the electrons around the atom start to oscillate with the same frequency as the incoming beam. In almost all directions we will have destructive interference, that is, the combining waves are out of phase and there is no resultant energy leaving the solid sample. However the atoms in a crystal are arranged in a regular pattern, and in a very few directions we will have constructive interference. The waves will be in phase and there will be well defined xray beams leaving the sample at various directions. Hence, a diffracted beam may be described as a beam composed of a large number of scattered rays mutually reinforcing one another. This model is complex to handle mathematically, and in day to day work we talk about x-ray reflections from a series of parallel planes inside the crystal. The orientation and interplanar spacing of these planes are defined by the three integers h, k, l called indices. A given set of planes with indices h, k, l cut the a- axis of the unit cell in h sections, the b axis in k sections and the c axis in l sections. A zero indicates that the planes are parallel to the corresponding axis. e.g. the 2, 2, 0 planes cut the a– and the b– axes in half, but are parallel to the c– axis. Structural Characterization of Thin Film by X-ray Diffraction(XRD) Bragg’s Diffraction : The relationship describing the angle at which a beam of X-rays of a particular wavelength diffracts from a crystalline surface was discovered by Sir William H. Bragg and Sir W. Lawrence Bragg and is known as Bragg’s Law nλ = 2dsinθ λ = wavelength of the x-ray θ = scattering angle n = integer representing the order of the diffraction peak. d = inter-plane distance of (i.e atoms, ions, molecules) Components 1- X-ray source 2- Device for restricting wavelength range “goniometer” 3- Sample holder 4- Radiation detector 5- Signal processor and readout Diffraction Methods: 1- Laue method The Laue method was the first diffraction method ever used. A beam of white radiation falls on a fixed single crystal. The Bragg angle theta is therefore fixed for every set of planes in the crystal, and each set picks out and diffracts that particular wavelength which satisfies the Bragg law for the particular values of the lattice distance d and the diffraction angle theta involved. There are two variants of the Laue method, depending on the relative positions of the source, crystal and film: transmission Laue method and back-reflection Laue method. 2-Powder method: The powder method is used to determine the value of the lattice parameters accurately. Lattice parameters are the magnitudes of the unit vectors a1, a2 and a3 which define the unit cell for the crystal. The crystal is finely grounded and the resulting powder containing thousands of crystallites are put into the monochromatic X-ray beam. A circle of film is used to record the diffraction pattern., as shown in Figure below 3-Rotating Crystal Method In the rotating crystal method, a single crystal is mounted with an axis normal to a monochromatic x-ray beam. A cylindrical film is placed around it and the crystal is rotated about the chosen axis. As the crystal rotates, sets of lattice planes will at some point make the correct Bragg angle for the monochromatic incident beam, and at that point a diffracted beam will be formed. The main application of the rotating crystal method is the determination of unknown crystal structures. Applications of X-Ray Diffraction: 1-Determinationof Crystal structure. 2-Phaseidentification/ transition. 3-Grainsize / micro-strain. 4-Texture/stress( i.e.polymer, fiber ). 5-Determination of thin film composition. 6-Industry Identification of archeological materials