poc3378-sup-0001-documentS1

Robust Hydrogen-bonded Capsules with Stability in Competitive Media
Konrad Tiefenbacher, b Kang-da Zhang, b Dariush Ajami b and Julius Rebek, Jr.
a,b a Department of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China.
The Skaggs Institute for Chemical Biology and the Department of Chemistry,
The Scripps Research Institute
10550 North Torrey Pines Road, La Jolla, CA 92037, United States.
Dedicated to Professor Charles Perrin
Supporting Information
1
Materials and General Methods
All reactions were carried out under an atmosphere of argon unless otherwise indicated.
Analytical thin-layer chromatography (TLC) was performed on Silicycle 60 F254 glass-baked plates. 1 H NMR and 13 C NMR spectra were recorded at 600 MHz and 150 MHz respectively, using a Bruker DRX-600 spectrometer equipped with a 5 mm QNP probe. Chemical shifts of
1 H NMR and 13 C NMR are given in ppm by using CHCl
3
or DMSO as references (7.26 ppm,
2.50 ppm respectively for 1 H spectrum, and 77.16 ppm, 39.52 ppm respectively for 13 C spectrum). Coupling constants (J) are reported in Hertz (Hz). Standard abbreviations indicating multiplicity were used as follows: s (singlet), b (broad), d (doublet), t (triplet), q
(quartet), m (multiplet). MALDI-TOF spectra and high-resolution mass spectra (HRMS) were recorded on an Applied Biosystems Voyager STR (2) apparatus and an Agilent ESI-TOF mass spectrometer respectively. Melting points were determined on a Mel-Temp II instrument
(Laboratory Devices, USA) and are uncorrected.
Anhydrous CH
2
Cl
2
, NEt
3
and Et
2
O were taken from a solvent drying system (SG
Water USA). All deuterated solvents were purchased from Cambridge Isotope Laboratories,
Inc.
2
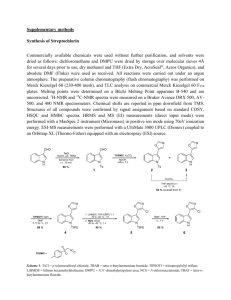
Tetra-oxobenzimidazole cavitand ( 1 )
A mixture of EtOH (100 mL) and HCl aq conc. (40 mL) was degassed by bubbling through
N
2
for 10 min. Octanitro cavitand 1 (2.00 g, 1.14 mmol) and SnCl
2
.2H
2
O (12.4 g, 54.7 mmol) was added and the mixture refluxed for 23h under Ar. After letting the white suspension cool to rt, the solid was filtered, washed with a solution of EtOH/HCl aq (100 mL/40 mL) and dried at 50°C (with stirring) under high vacuum for 24 h to give the octamine hydrochloride as a white solid (2.1 g). It was directly used for the next step.
The crude octamine hydrochloride (2.1 g, th.: 1.14 mmol) was suspended in THF (120 mL), triphosgene (1.49 g, 5.02 mmol) and Et
3
N (4.6 mL, 33.0 mmol) added and stirred for 19 h.
Water (2 mL) was added and the white suspension concentrated under vacuum. After the addition of water (100 mL) and sonication for 30 min., the solid was filtered and washed with water (3x). It was resuspended in MeOH (150 mL) and refluxed for 45 min., filtered hot and washed with MeOH (3x). Traces of solvents were removed under vacuum to yield cavitand 1
(1.64 g, 88%) as a ivory solid.
1 H NMR (600 MHz, d6-DMSO, 343 K) δ=9.89 (s, 8H), 7.59 (s, 4H), 7.57 (s, 4H), 7.24 (s,
8H), 5.56 (t, J = 8.3 Hz, 4H), 2.33-2.27 (m, 8H), 1.44-1.34 (m, 8H), 1.34-1.22 (m, 64H), 0.88
(t, J = 6.8 Hz, 12H).
13 C NMR (150 MHz, d6-DMSO, 350 K) δ= 155.6, 155.0, 145.8, 134.5, 126.5, 124.2, 116.2,
103.7, 32.6, 30.8, 28.7, 28.5 (br), 28.1, 27.3, 21.5, 13.2.
MALDI-TOF calcd. for C100H121N8O12+ [M + H] + : 1626, found: 1626.
MALDI-TOF calcd. for C100H120N8O12Na+ [M + Na] + : 1648, found: 1648.
Mp : > 220 ° C
IR ṽ = 2920, 2851, 1715, 1484, 1158, 1112. cm -1
3
General procedure for the encapsulation studies
To a mixture of cavitand 1 (2.0 mg, 0.0012 mmol) and guest (0.006 mmol) in a NMR-tube was added d12-mesitylene (0.50 mL) and methanol (25 uL). The solution was sonicated for 3h.
Boc-protected TEG-tetra-oxobenzimidazole cavitand ( 13 )
To a solution of TEG-octanitrocavitand 2 12 (300 mg, 0.140 mmol) in THF (5.6 mL) was added Raney-Ni (ca. 200 mg, washed with THF 5x) suspended in THF (1 mL). The flask was filled with hydrogen by vacuum/hydrogen-cycles (5x) and stirred under a hydrogenatmosphere at 45 ° C for 16 h. The solution is transferred to a second flask under Argon
(Raney-Ni removed with help of a magnet), concentrated under vacuum at rt and dried under high vacuum to remove traces of water, to give crude the crude TEG-octaamine cavitand 14
(oxidation-sensitive!), which was immediately used for the next step.
The crude TEG-octaamine cavitand 14 (th.: 0.140 mmol) was dissolved in THF (15 mL), triphosgene (208 mg, 0.700 mmol) and Et
3
N (254 uL, 1.82 mmol) added and stirred for
23 h. Water (0.1 mL) was added, the suspension concentrated under vacuum and dried under high vacuum to remove traces of water. The dried material was suspended in THF (18 mL),
Et
3
N (0.68 mL, 4.89 mmol), Boc
2
O (611 mg, 2.80 mmol) and DMAP (137 mg, 1.12 mmol) added. After stirring for 65 h, the solvents were removed under vacuum, to give a yellow solid, which was purified by column chromatography (100 mL silica gel) using
CH
2
Cl
2
/MeOH 95:5 as an eluent, to yield cavitand 13 (159 mg, 40% over 3 steps) as a colorless film.
1 H NMR (600 MHz, CDCl
3
) δ=7.68 (s, 8H), 6.89 (s, 4H), 6.78 (s, 4H), 4.59 (t, J = 8.1 Hz,
4
4H), 3.64-3.49 (m, 64H), 3.45 (t, J = 6.6 Hz, 8H), 3.36 (s, 12H), 2.12-2.04 (m, 8H), 1.68-1.62
(m, 8H), 1.61 (s, 72H).
13 C NMR (150 MHz, CDCl
3
) δ=154.1, 148.2, 147.3, 145.0, 132.2, 123.8, 122.6, 113.7, 108.3,
85.8, 72.1, 70.7, 70.7, 70.7, 70.6, 70.2, 59.1, 34.6, 28.2, 27.4.
MALDI-TOF calcd. for C104H128N8O32Na+ [M – 8 Boc + Na] + : 2024, found: 2024.
IR ṽ = 2868, 1789, 1737, 1479, 1333, 1112. cm -1
TEG-Tetra-oxobenzimidazole cavitand ( 1b )
Cavitand 13 (108 mg, 0.0385 mmol) was dissolved in MeOH/HCl (8 mL; prepared by bubbling HCl-gas through MeOH for 10 min.) and stirred for 24 h. The mixture was concentrated under vacuum, water (10 mL) added and sonciated to give a off-white solid. It was filtered, washed with water (3x) and dried in a desiccator to give TEG-cavitand 1b (46 mg, 60%).
1 H NMR (600 MHz, d6-DMSO) δ=10.11 (s, 8H), 7.76 (s, 4H), 7.64 (s, 4H), 7.40 (s, 8H),
5.52 (t, J = 8.4 Hz, 4H), 3.56-3.44 (m, 64H), 3.43- 3.38 (s, 8H), 3.23 (s, 12H), 2.33 (q, J = 7.7
Hz, 8H), 1.45 (m, 8H).
13 C NMR (150 MHz, d6-acetone) δ=161.1, 156.7, 148.8, 136.5, 128.0, 125.6, 117.4, 107.0,
72.7, 71.4, 71.3, 71.1, 71.0, 58.9, 34.2, 30.6, 29.2.
MALDI-TOF calcd. for C104H128N8O32Na+ [M + Na] + : 2024, found: 2024.
MALDI-TOF calcd. for C104H129N8O32+ [M + H] + : 2002, found: 2002.
IR ṽ = 2867, 1712, 1484, 1102, 1015, 807. cm -1
5
References
(1) Choi, H. J.; Park, Y. S.; Cho, C. S.; Koh, K.; Kim, S. H.; Paek, K. Org. Lett.
2004 , 6 ,
4431. The material was additionally purified by refluxing it in MeCN (50 mL/g). After filtration, the material was washed with MeCN (3x) and solvent traces removed under vacuum).
(2) Lledo, A.; Rebek, J. Chem. Commun.
2010 , 46 , 8630.
6