Entereg Patient Case
advertisement

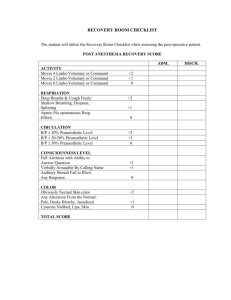
Patient: MJ Age: 83 CrCl: 55 mL/min Max Whitney, PharmD Surgical Rotation Case Conference August 8, 2014 Gender: F Weight: 59 kg Height: 5’ Allergies: Aspirin (stomach cramps) Admit Date: 7/30/2014 Discharge Date: 8/4/2014 Reason for Admission: Abdominoperineal resection History of Present Illness: MJ saw PCP 11/2013 for chronic anemia and thrombocytopenia where GI endoscopic evaluations were recommended but not followed through by patient Patient presented to ED 7/13/2014 after large, bloody BM at home Colonoscopy performed on admission which revealed lower rectal cancer Family opted out of chemotherapy and radiation; therefore, surgical intervention is being performed to excise the large tumor during this admission Past Medical History: GERD Osteoarthritis Anemia/Chronic Thrombocytopenia Alzheimer’s Disease Past Surgical History: TKA Sinus surgery Appendectomy Tonsillectomy Pertinent Medications: Drug Alvimopan 12 mg x 1 pre-op, then twice daily beginning 7/31 AM Hydromorphone 0.2-1mg IV every 2 hours as needed Hydrocodone/APAP 5/525 mg 1-2 tabs PO every 4 hours as needed Start Date 7/30 7/30 8/2 End Date 8/4 8/2 8/4 Indication Prevention of post-op ileus Severe post-operative pain Moderate post-operative pain Post-Operative Ileus Prevention Management Subjective/Objective: Post-operative recovery was uneventful day-to-day MJ was started on thin oral liquids 7/30 post-op and was gradually advanced Stool output from the colostomy bag was first seen on post-operative day #3 Pain scores ranged from 2-5/10 and was well-controlled with IV hydromorphone and oral hydrocone/APAP Assessment/Plan: Patient has low level of dementia and was discharged to home with daughter MJ was able to discharge on post-operative day #6 in stable condition and intact colostomy site Patient resumed home medications and was given a script for oxycodone/APAP 5/325 mg Follow-up with surgeon as an outpatient one week from discharge date Entereg® (alvimopan) Dosing1,2 Indications1,2 Black Box Warning1,2 Contraindications1,2 Warnings/Precautions1,2 MOA1-6 Pharmacokinetics1,2 Adverse Drug Reactions1-6 Additional Information Resources Adult: 12 mg 30 minutes to 5 hours prior to surgery, followed by 12 mg twice daily beginning the day after surgery for up to 7 days or until hospital discharge. MAX 15 doses. Adjustments: No dose adjustments necessary Post-operative ileus Following partial large or small bowel resection surgery An increased risk of myocardial infarction was seen in alvimopan-treated patients compared with placebo when duration of therapy extended beyond 1 month. Opioid use for more than 7 days immediately prior to taking alvimopan. Increase risk of MI (with 1-4 months of therapy) Complete GI obstruction Hepatic impairment: increased ADR risk d/t potentially increased concentrations ESRD: use not recommended Mu-opioid receptor antagonist within the GI tract, thereby blocking the peripheral effects of opioids. Absorption 6% bioavailability; food decreases rate and extent of absorption. Distribution Vd= 30 L; 80% bound to albumin. Metabolism Metabolized to active metabolite in the intestinal wall by normal flora. Excretion Primarily via the bile and stool. 35% excreted by kidneys. Half-life 10-17 hours Indigestion (1.5%) diarrhea, gastrointestinal pain, cramping, nausea Myocardial Infarction One study found the mean total hospital cost was $12,865 for alvimopan Cost patients compared to $13,905 for controls (P=.033)7 Drug Expect interactions with known p-glycoprotein inhibitors such as Interactions amiodarone, diltiazem, cyclosporine, itraconazole, and verapamil. Future May alvimopan be discontinued after the first successful BM? Considerations Will use be expanded to pediatrics, opioid-induced constipation? 1 Product Information: ENTEREG(R) oral capsules, alvimopan oral capsules. Cubist Pharmaceuticals, Inc. (per manufacturer), Lexington, MA, 2013. 2. Micromedex Healthcare Series. DrugDex. Greenwood Village, CO: Truven Health Analytics, 2014. http://www.micromedex.com/. Accessed August 5, 2014. 3. Tan E.K., Cornish J., Darzi A.W., et al: Meta-analysis: Alvimopan vs. placebo in the treatment of post-operative ileus. Aliment Pharmacol Ther 2007; 25(1):47-57. 4. Wolff BG, Michelassi F, Gerkin TM, et al: Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg 2004; 240(4):728-735. 5. Taguchi A, Sharma N, Saleem RM, et al: Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med 2001; 345(13):935-940. 6. Vaughan-Saw PG, Fecher IC, Harris S, Knight JS. A meta-analysis of the effectiveness of the opioid receptor antagonist alvimopan in reducing hospital length of stay and time to GI recovery in patients enrolled in a standardized accelerated recovery program after abdominal surgery. Dis Colon Rectum 2012 May;55(5):611-620. 7. Poston S, Broder MS, Gibbons MM, et al. Impact of alvimopan on hospital costs after bowel resection. P.T. 2011 April;36(4):209-220. 8. Akca O, Doufas AG, & Sessler DI: Use of selective opiate receptor inhibitors to prevent postoperative ileus. Minerva Anestesiol 2002; 68(4):162-165.