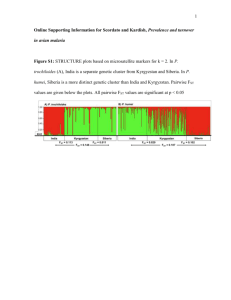
jfb12677-sup-0001
advertisement

1 Supporting information: Genotyping and classification 2 Microsatellite typing 3 Caudal fin samples (stored on 96% ethanol) were lysed overnight at 55ºC in 200 µl lysisbuffer 4 with 4 µl of 20 mg ml-1 proteinase K. For part of the samples DNA was extracted using 5 genemole: samples were centrifuged for 5 min at 14000 rpm, 100 µl supernatant was 6 transferred to gene mole tubes, and DNA was eluted in 100 µl H2O. Another part was 7 extracted using chelex (Walsh et al., 1991): Overnight lysis of caudal fin tissue was done in 8 300 µl 5% chelex with 9 µl 20 mg ml-1 proteinase K, after which samples were vortexed, 9 centrifuged for 5 min at 14000 rpm and 200 µl supernatant was transferred to new tubes. For 10 the rest of samples, DNA was extracted using the Qiagen DNA isolation kit with spin- 11 columns, following the instructions for tissue samples. Five µl of the resulting DNA extract 12 was transferred to 96-well plates and diluted 10x. PCR amplification was carried out in 10 µl 13 volumes consisting of 2 µl DNA template, 0.9 µl MgCl, 0.5 µl dNTP, 0.15 µl Thermostart 14 polymerase, 1 µl Thermostart buffer and primers. The primers were grouped into 3 15 multiplexes (A, B and 1), and forward primers were fluorescently labeled with FAM, NED or 16 VIC. The reference samples were typed for all 3 multiplexes, and based on differentiating 17 power, multiplexes A and 1 were chosen for the samples to be classified. Mix A included 0.4 18 µl (100µM forward and reverse) Ssa289 (FAM), 0.2 µl Ssa14 (FAM) (McConnell et al., 19 1995), 0.1 µl Ssa197 (FAM), 0.2 µl Ssa171 (FAM) (O’Reilly et al., 1996) and 0.2 µl Ssa408 20 (NED; Cairney et al., 2000). Mix 1 included 0.035 µl Sssp2210 (FAM), 0,035 µl SsspG7 21 (PET), 0.17 µl Sp2201 (PET) (Paterson et al., 2004), 0.2 µl SsaD144 (NED), 0.25 µl 22 SsaD157 (NED) (King et al. 2005) and 0.1 µl Ssa202 (FAM; O’Reilly et al., 1996) (See 23 Gilbey et al. (2004) table 1 for base sequence, annealing temperature and GenBank accession 24 code of many of the primers). Based on this, seven fish could initially not be classified with 1 25 certainty, and were additionally genotyped for mix B. Mix B included 0.05 µl u20.19 (FAM; 26 Sanchez et al., 1996), 0.3 µl Ssosl85 (NED; Slettan et al., 1995) and 0.3 µl Ssosl438 (VIC; 27 Slettan et al., 1996). 28 29 Analysis for length polymorphism was performed on an applied biosystems ABI 30 330XL using size standard GS500LIZ. Alleles were automatically called and manually 31 checked in GeneMapper v. 4.0. 32 Microsatellite Toolkit (Park, 2001) and by checking for outliers in the principal component 33 plot in Genetix v 4.05 (Belkhir et al., 1996-2004). The mean number of alleles per marker 34 was 9. Screening for errors was done using the Excel add-in 35 36 Classification 37 Since genotypes of the parents of either group were not available, 26 unrelated Aqua 38 Gen fish and 24 unrelated Imsa fish from the same year classes as the parents were used as 39 reference groups for classification. Identical results were obtained from GeneClass 2 (Piry et 40 al., 2004) and Structure 2.3.3 (Pritchard et al., 2000) using a threshold of 95%. All 7 of the 41 fish from the channels could be classified to either group, but 13 fish from the unconfined 42 stream channels could not be classified with certainty, of which 11 were genotyped for 3 43 markers or less, despite repeated attempts. 44 45 46 References 47 48 2 49 Belkhir K., Borsa P., Chikhi L., Raufaste N. & Bonhomme F. (1996-2004). GENETIX 4.05, logiciel 50 sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, 51 Interactions, CNRS UMR 5171, Université de Montpellier II, Montpellier (France). 52 Cairney M., Taggart J. B. & Hoyheim B. (2000). Characterization of microsatellite and minisatellite 53 loci in Atlantic salmon (Salmo salar L.) and cross-species amplification in other salmonids. 54 Molecular Ecology 9, 2175–2178. 55 56 Gilbey, J., Verspoor, E., McLay, A., & Houlihan, D. (2004). A microsatellite linkage map for Atlantic salmon (Salmo salar). Animal Genetics, 35, 98-105. doi: 10.1111/j.1365-2052.2004.01091.x 57 King T. L., Eackles M. S. & Letcher B. H. (2005). Microsatellite DNA markers for the study of 58 Atlantic salmon (Salmo salar) kinship, population structure, and mixed-fishery analyses. 59 Molecular Ecology Notes 5, 130–132. 60 McConnell, S. K., O’Reilly, P., Hamilton, L., Wright, J. M. & Bentzen, P. (1995). Polymorphic 61 microsatellite loci from Atlantic salmon (Salmo salar): genetic differentiation of North 62 American and European populations. Canadian Journal of Fisheries and Aquatic Sciences 52, 63 1863–1872. 64 O’Reilly, P. T., Hamilton, L. C., McConnell, S. K. & Wright, J. M. (1996). Rapid analysis of genetic 65 variation in Atlantic salmon (Salmo salar) by PCR multiplexing of dinucleotide and 66 tetranucleotide microsatellites. Canadian Journal of Fisheries and Aquatic Sciences 53, 2292– 67 2298. 68 69 70 Park, S. D. E. (2001). Trypanotolerance in west african cattle and the population genetic effects of selection. Ph.D. thesis, University of Dublin. Paterson, S., Piertney, S. B., Knox, D., Gilbey, J., & Verspoor, E. (2004). Characterization and PCR 71 multiplexing of novel highly variable tetranucleotide Atlantic salmon (Salmo salar L.) 72 microsatellites. Molecular Ecology Notes 4, 160-162. doi: 10.1111/j.1471-8286.2004.00598.x 73 Piry S., Alapetite A., Cornuet, J.-M., Paetkau D., Baudouin, L. & Estoup, A. (2004). GeneClass2: A 74 Software for Genetic Assignment and First-Generation Migrant Detection. Journal of Heredity 75 95, 536-539. 3 76 77 78 Pritchard J.K., M Stephens M. & Donnelly P.J. (2000). Inference of population structure using multilocus genotype data. Genetics 155, 945–959. Sanchez J. A., C. Clabby, D. Ramos, G. Blanco, F. Flavin, E. Vazquez, & R. Powel . (1996). Protein 79 and microsatellite single locus variability in Salmo salar L (Atlantic salmon). Heredity 77, 80 423-432. 81 82 83 84 85 86 Slettan A., Olsaker I. & Lie O. (1995) Atlantic salmon, Salmo salar, microsatellites at the SSOSL25, SSOSL85, SSOSL311, SSOSL417 loci. Animal Genetics 26, 277–85. Slettan A., Olsaker I. & Lie O. (1996) Polymorphic Atlantic salmon, Salmo salar L., microsatellites at the SSOSL438, SSOSL439 and SSOSL444 loci. Animal Genetics 27, 57–64. Walsh, P. S., Metzger, D. A., & Higuchi, R. (1991). Chelex-100 as a medium for simple extraction of DNA for pcr-based typing from forensic material. Biotechniques 10, 506-513. 87 4