Why O2 and temperature
advertisement

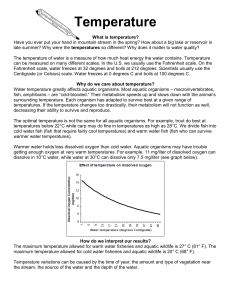
Dissolved oxygen in any water source, such as a lake or a river, is crucial to all living organisms in the water. Oxygen gets into the water mainly by three factors; diffusion from surrounding air, aeration of water that has been stirred up by something such as a water fall, and as a waste product from photosynthesis. Dissolved oxygen also affects the taste of drinking water. The more dissolved oxygen, the better it will taste. However, high DO content will speed up the corrosion process in things such as pipes. That is why many manufacturers tend to use low DO content water. Dissolved oxygen content should never exceed 110 percent. This can harm aquatic life. Fish living in such conditions may get the “gas bubble disease” though it is rare. Adequate dissolved oxygen is necessary for good water quality. Oxygen is a necessary element to all forms of life. Natural stream purification processes require adequate oxygen levels in order to provide for all oxygen dependent species. As dissolved oxygen levels in water drop below 5.0 mg/l, aquatic life is put under stress. The lower the concentration, the greater the stress. Oxygen levels that remain below 1-2 mg/l for a few hours can result in large fish kills. Oxygen is one of the best indicators for water quality. Temperature is also another important factor for water quality. It affects both chemical and biological characteristics of water. When the temperature is too warm, there tends to be a lot of aquatic life such as animals and bacteria that overpopulates and uses an excessive amount of oxygen. Temperature also impacts water by thermal pollution. Thermal pollution is the introduction of water that is warmer than the body of water it is flowing into. Thermal pollution may occur from factories dumping hot water to cool equipment into streams. Another factor may come from urban runoff. This is when water is heated from running over parking lots or roads. Deforestation also affects thermal pollution by reducing the amount of shade. Warm water is less capable of holding dissolved oxygen. For this reason, temperature should be measured at the same place within the stream at which dissolved oxygen is measured.