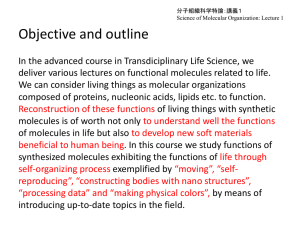
Simple Molar Conversions
advertisement
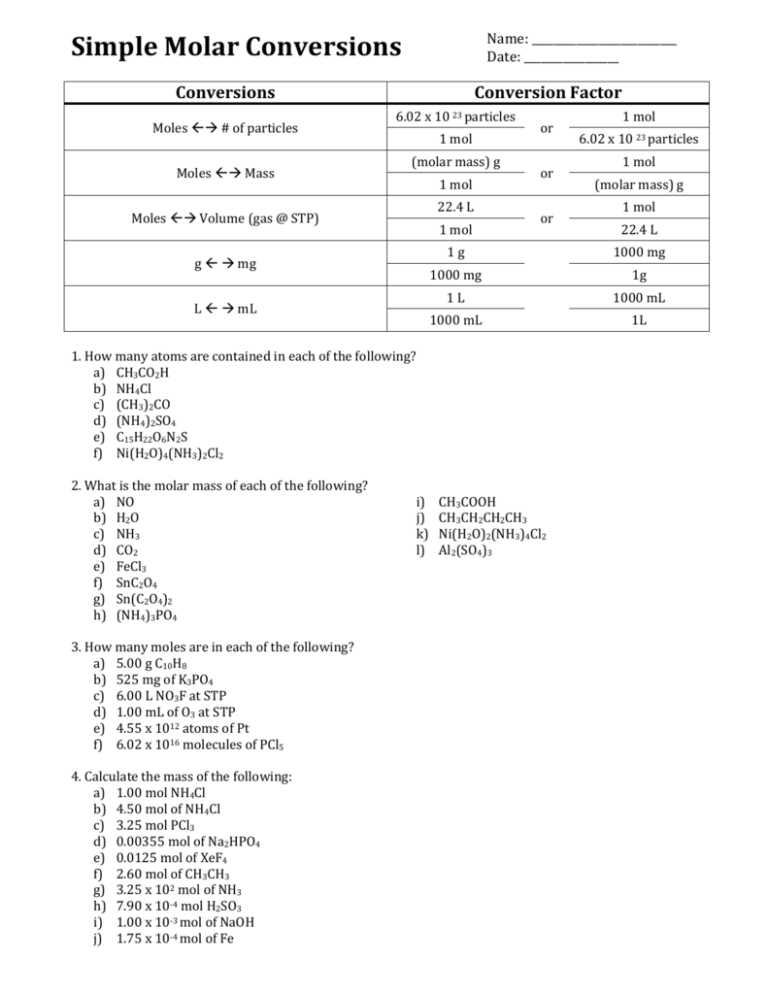
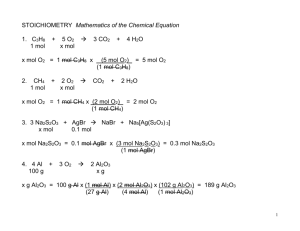
Simple Molar Conversions Name: __________________________ Date: _________________ Conversions Moles # of particles Moles Mass Conversion Factor 6.02 x 10 23 particles 1 mol (molar mass) g 1 mol 22.4 L Moles Volume (gas @ STP) 1 mol g mg L mL or or or 3. How many moles are in each of the following? a) 5.00 g C10H8 b) 525 mg of K3PO4 c) 6.00 L NO3F at STP d) 1.00 mL of O3 at STP e) 4.55 x 1012 atoms of Pt f) 6.02 x 1016 molecules of PCl5 4. Calculate the mass of the following: a) 1.00 mol NH4Cl b) 4.50 mol of NH4Cl c) 3.25 mol PCl3 d) 0.00355 mol of Na2HPO4 e) 0.0125 mol of XeF4 f) 2.60 mol of CH3CH3 g) 3.25 x 102 mol of NH3 h) 7.90 x 10-4 mol H2SO3 i) 1.00 x 10-3 mol of NaOH j) 1.75 x 10-4 mol of Fe i) j) k) l) 6.02 x 10 23 particles 1 mol (molar mass) g 1 mol 22.4 L 1g 1000 mg 1000 mg 1g 1L 1000 mL 1000 mL 1L 1. How many atoms are contained in each of the following? a) CH3CO2H b) NH4Cl c) (CH3)2CO d) (NH4)2SO4 e) C15H22O6N2S f) Ni(H2O)4(NH3)2Cl2 2. What is the molar mass of each of the following? a) NO b) H2O c) NH3 d) CO2 e) FeCl3 f) SnC2O4 g) Sn(C2O4)2 h) (NH4)3PO4 1 mol CH3COOH CH3CH2CH2CH3 Ni(H2O)2(NH3)4Cl2 Al2(SO4)3 5. Calculate the volume of the following gases at STP: a) 0.235 mol of B2H6 b) 9.36 mol of SiH4 c) 2.55 x 103 mol of C2H6 6. Calculate the mass of each of the following: a) 0.125 mol of CO2 at STP b) 5.48 mol of solid FeCl3 c) 6.54 x 10-4 mol of HCN at STP d) 15.4 mol of solid Ni(OH)2 7. (a) How many atoms are in 2 molecules of Hg(IO3)2? (b) What volume at STP is occupied by 1.45 x 1030 molecules of COF2 gas? (c) How many molecules are there in 64.0 g of FeS? (d) How many moles are in 25.0 mL of HCN at STP? (e) What volume at STP is occupied by 43.5 g of ClF3? (f) How many moles are in 2.75 x 10 23 atoms of Fe? (g) How many molecules are there in 125 mL of NOCl at STP? (h) What is the mass of 3.01 x 1022 atoms of Pt? (i) What is the mass of 25.0 mL of Kr at STP? (j)What is the molar mass of 00139 mol of a substance having a mass of 0.888 g? (k) What is the density of acetic acid, CH3COOH, if 0.250 mol of CH3COOH has a volume of 14.3 mL? (l) How many moles are in 85.0 mg of CuSCN? (m) If 135 L of cyanogen gas has a mass of 313 g at STP, what is the molar mass of cyanogen? ANSWERS: 1. a) 8 b) 6 c) 10 d) 15 e) 46 f) 23 2. a) 30.0 g/mol b) 18.0 g/mol c) 17.0 g/mol d) 44.0 g/mol e) 162.3 g/mol f) 206.7 g/mol g) 294.7 g/mol h) 149.0 g/mol i) 60.0 g/mol j) 58.0 g/mol k) 233.7 g/mol l) 342.3 g/mol 3. a) 0.0391 mol b) 0.00247 mol c) 0.268 mol d) 4.46 x 10-5 mol e) 7.56 x 10-12 mol f) 1.00 x 10-7 mol 4. a) 53.5g b) 241g c) 447g d) 0.504g e) 2.59g f) 78.0g g) 5.53 x 103g h) 0.0649g i) 0.0400g j) 9.77 x 10-3g 5. a) 5.26 L b) 2.10 x 102 L c) 5.71 x 104 L 6. a) 5.50g b) 889 g c) 0.0177 g d) 1.43 x 103 g 7. a) 18 atoms b) 5.40 x 10 7 L c) 4.38 x 10 23 molecules d) 1.12 x 10-3 mol e) 10.5 L f) 0.457 mol g) 3.36 x 1021 molecules h) 9.76 g i) 0.0935 g j) 63.9 g/mol k) 1.05 g/mL l) 6.99 x 10 -4 mol m) 51.9 g/mol