February 4, 2013
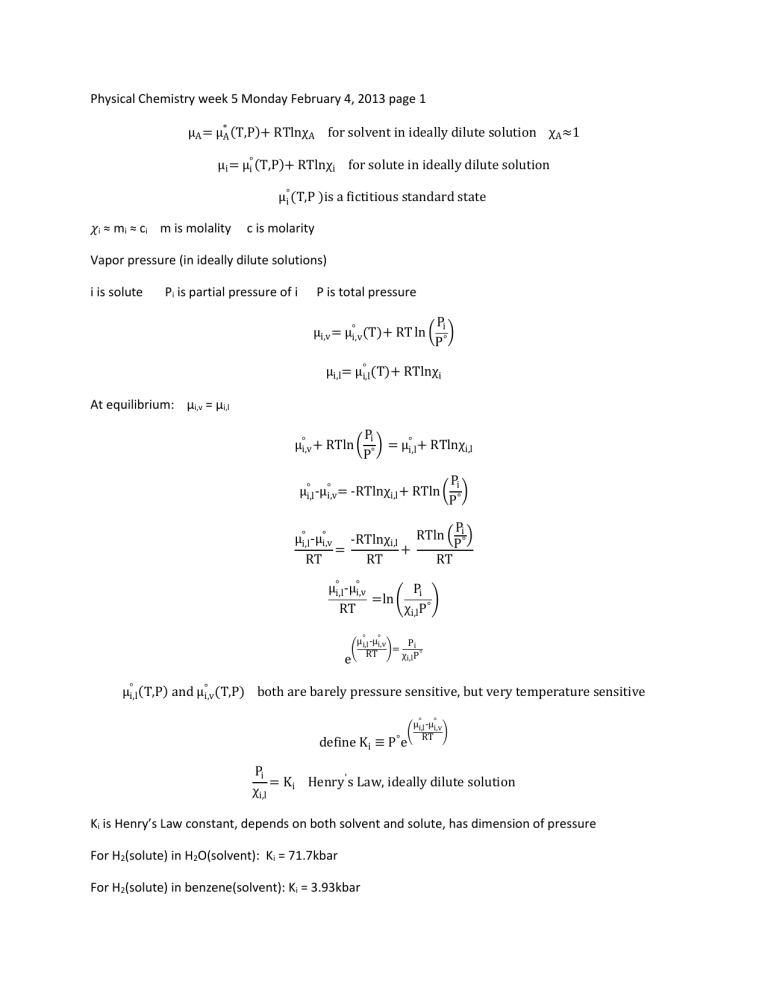
Physical Chemistry week 5 Monday February 4, 2013 page 1
μ
A
= μ
*
A
(T,P)+ RTlnχ
A
for solvent in ideally dilute solution χ
A
≈1
μ i
= μ i
°
(T,P)+ RTlnχ i
for solute in ideally dilute solution
μ i
°
(T,P )is a fictitious standard state 𝜒 i
≈ m i
≈ c i
m is molality c is molarity
Vapor pressure (in ideally dilute solutions) i is solute P i
is partial pressure of i P is total pressure
μ i,v
= μ
° i,v
P
(T)+ RT ln (
P i
°
)
μ i,l
= μ
° i,l
(T)+ RTlnχ i
At equilibrium: µ i,v
= µ i,l
μ
° i,v
P
+ RTln (
P i
°
) = μ
° i,l
+ RTlnχ i,l
μ
° i,l
-μ
° i,v
= -RTlnχ i,l
P
+ RTln (
P i
°
)
μ
° i,l
-μ
° i,v
RT
=
-RTlnχ i,l
RT
+
RTln (
P
RT
P i
°
)
μ
° i,l
-μ
° i,v
RT
P
=ln (
χ i,l i
P °
) e
(
μ
° i,l
-μ
° i,v
RT )
=
χ
P i i,l
P °
μ
° i,l
(T,P) and μ ° i,v
(T,P) both are barely pressure sensitive, but very temperature sensitive define K i
≡ P ° e
(
μ
° i,l
-μ
° i,v
RT )
P i
χ i,l
= K i
Henry
' s Law, ideally dilute solution
K i
is Henry’s Law constant, depends on both solvent and solute, has dimension of pressure
For H
2
(solute) in H
2
O(solvent): K i
= 71.7kbar
For H
2
(solute) in benzene(solvent): K i
= 3.93kbar
Lower K i
→ higher solubility
χ i,l
=
P i
K i
CO, Ar, O
2
, N
2
, CH
4
, C
2
H
6
also more soluble in benzene than in water
P i
= K i 𝜒 i,l
solute obeys Henry’s Law in ideally dilute solution
P
A
= 𝜒
A
P
A
* solvent obeys Raoult’s Law variation of gas solubility with temperature
Generally, as T↑, solubility↓ in H
2
O
Organic solvent: as T↑, solubility ↑
Henry’s Law applies to nonelectrolytes.
HCl(g) + H
2
O → H
3
O + + Cl so Henry’s Law doesn’t apply
NH
3
(g) + H
2
O
⇌
NH
4
+ + OH -
Consider a graph with mole fraction on the vertical axis and pressure on the horizontal axis. A diagonal line from the origin up and right would be Henry’s Law and have a slope of K i
-1 . Hydrogen gas would start at the origin and follow Henry’s Law for a while, then curve down. Nitrogen gas would also start at the origin and follow Henry’s Law for a while, then curve down sooner than hydrogen. This means
Henry’s Law is a good approximation for either at low pressure. They both curve down eventually because K i
isn’t really constant with increasing P.
Consider a graph with pressure on the vertical axis and mole fraction on the horizontal axis. One straight line goes up and right from the origin. This is the curve for an ideally dilute solution following
Henry’s Law. At the right side of this curve is where K i
= P i
. A second curve also starts at the origin and goes up to the right side. This is the curve for Raoult’s Law. At the right side of this curve is where P
A
=
P * . As mole fraction of solute or solvent goes to 1, K i
=Pi and P
A
= P*. A third curve is between those two. This is the actual pressure. The deviation on the left going up is from Henry’s Law. The deviation on the right going down is from Raoult’s Law.
Consider a graph with pressure on the vertical axis and mole fraction on the horizontal axis. The left side is pure acetone(abbreviated ace) and the right side is pure chloroform(abbreviated chl). At the left side is a pressure corresponding to pure acetone, P ace
* . At the right side is a pressure corresponding to pure chloroform, P chl
* . A dotted line goes from the bottom left to the right side at P chl
* . This is what the ideal partial pressure of chloroform would be. A curve sagging below it with the same endpoints is the actual partial pressure of chloroform. This sagging is called negative deviation. A second dotted line goes from the bottom right to the left side at P ace
* . This line represents what the ideal partial pressure of acetone would be. A curve sagging below it with the same endpoints is the actual partial pressure of acetone. This sagging is also negative deviation. A straight line connects the two sides from P ace
* to P chl
* .
This is what the total pressure would be if it were an ideal solution. A curve sags down below it with the same endpoints. This is the actual total pressure. The sagging is negative deviation. Hydrogen bonding is when you have a H next to an O, N, or F in one molecule bonded to an O,N, or F in another molecule.
Acetone and chloroform don’t technically meet this definition of hydrogen bonding, but the C in chloroform is very electron deficient, causing the H-C bond to be very polar, which bonds well with the unbound electrons in the O of acetone. Since acetone and chloroform bond to each other better than to themselves, mixing them lowers the vapor pressure, causing negative deviation.
Consider a graph with pressure on the vertical axis and mole fraction of carbon disulfide on the horizontal axis. A straight line goes from the origin to the right side at a pressure P
CS2
* . This is what the pressure would be if the solution were ideal. A curve rises above this line with the same endpoints. This is the actual partial pressure of the CS
2
in acetone. This bulge is called positive deviation. Another straight line goes from the bottom right to the left side at pressure P ace
* . This line is what the partial pressure of the acetone would be if the solution were idea. A curve bulges above this line with the same endpoints. This bulge is called positive deviation. A straight line connects the left side at P ace
* to the right side at P
CS2
* . This is what the total pressure of the solution would be if it were behaving ideally. A curve bulges up above this. This is the actual pressure of the solution. It bulges up due to positive deviation. P > P ace
* + P
CS2
* . The positive deviation happens because CS
2
is nonpolar but acetone is polar.