porphyrin synthesis
advertisement
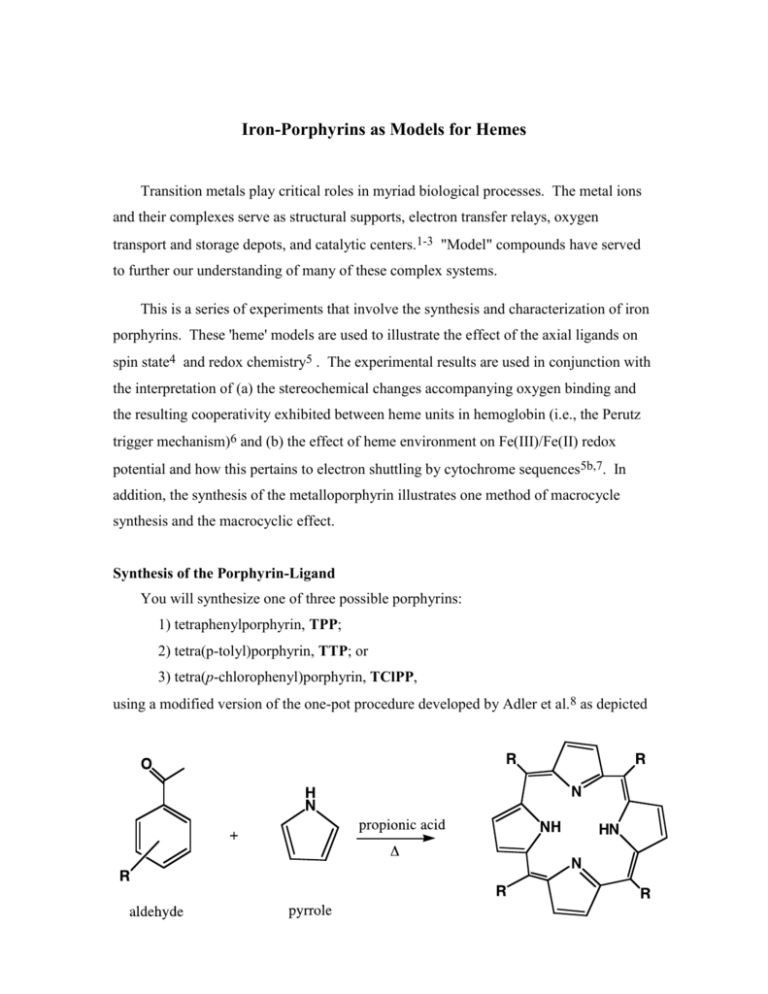
Iron-Porphyrins as Models for Hemes Transition metals play critical roles in myriad biological processes. The metal ions and their complexes serve as structural supports, electron transfer relays, oxygen transport and storage depots, and catalytic centers.1-3 "Model" compounds have served to further our understanding of many of these complex systems. This is a series of experiments that involve the synthesis and characterization of iron porphyrins. These 'heme' models are used to illustrate the effect of the axial ligands on spin state4 and redox chemistry5 . The experimental results are used in conjunction with the interpretation of (a) the stereochemical changes accompanying oxygen binding and the resulting cooperativity exhibited between heme units in hemoglobin (i.e., the Perutz trigger mechanism)6 and (b) the effect of heme environment on Fe(III)/Fe(II) redox potential and how this pertains to electron shuttling by cytochrome sequences5b,7. In addition, the synthesis of the metalloporphyrin illustrates one method of macrocycle synthesis and the macrocyclic effect. Synthesis of the Porphyrin-Ligand You will synthesize one of three possible porphyrins: 1) tetraphenylporphyrin, TPP; 2) tetra(p-tolyl)porphyrin, TTP; or 3) tetra(p-chlorophenyl)porphyrin, TClPP, using a modified version of the one-pot procedure developed by Adler et al.8 as depicted below Procedure for the synthesis of a tetra-substituted porphyrin **All manipulations including isolation of the product must be performed in a hood.** A stir bar and 0.3L propionic acid are added to a 500-mL three-neck, round-bottom flask fitted with a reflux condenser. Put the addition funnel in the center neck, the condensor on a side neck and a septum on the third neck. The acid is brought to a reflux and 0.1 mol of the appropriate substituted benzaldehyde is added. Quantities of aldehydes: benzaldehyde [for TPP synthesis] = 10.16 mL p-tolualdehyde [for TTP synthesis] = 11.8 mL 4-chlorobenzaldehyde [for TClPP synthesis] = 14.06 g To the refluxing solution, 10 mL (0.1 mol) freshly distilled pyrrole is cautiously added via a dropping funnel. A recommended rate is about 1 drop/sec. Caution! The condensation reaction of the pyrrole with the aldehyde is extremely exothermic, and the rate of pyrrole addition must be continuously monitored. The solution is refluxed for 30 min, cooled to ambient temperature, and chilled in an ice bath before vacuum filtering off the purple, crystalline product. [Note: Filtrations can be slow. Try adding small volumes of the reaction solution, rather than dumping the entire amount into the funnel. Also use of a coarse 40-60 mL fritted funnel for filtering the product.] The product is washed with boiling water (about 500 mL) until free from the odor of propionic acid. Typical yields should be in the range of 10-20%. The crude product is of sufficient purity for use in the metallation reaction. WASTE. The reaction solution filtrate goes in the provided container in the hood. The washings may be put down the drain.