Exam 3
advertisement

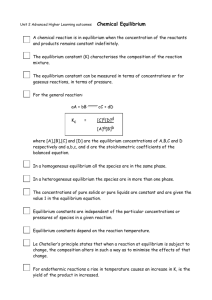
Exam 3: Thermodynamics, Kinetics, Equilibrium and Acid-Base Chemistry 12/2/15 Name Directions: Show all work. Use the back side of your exam for extra space as needed. 1. Why do reactions seek to approach equilibrium, rather than proceeding to completion in a given direction? (6) 2. What is Gibbs Free Energy? How can its value be used to predict the spontaneity of a reaction? (6) 3. Describe all three laws of thermodynamics. (6) 4. List 3 ways that the rate of a reaction can be changed (no explanations needed). (3) 5. What are the necessary thermodynamic conditions required for a reaction to be spontaneous: a.) At low temperatures only? (3) b.) At high temperatures only? (3) c.) At all temperatures? (3) 6. What is a buffer? How does it work? (5) 7. List the strong acids. How do strong acids and weak acids differ? (5) 8. Write out the balanced equilibrium reaction between dimethylamine, (CH 3)2NH, and water. Label the acid, base, and the conjugates. Then, assuming an initial dimethylamine concentration of 0.1M, determine the pH of the solution given that Kb = 5.4 x 10-5M. (15) 9. 𝑁2 (𝑎𝑞) + 𝑂2 (𝑎𝑞) ⇌ 2𝑁𝑂 (𝑎𝑞) 𝐾 = 0.09 a.) Consider the following initial concentrations: [𝑵𝟐 ] = [𝑶𝟐 ] = 𝟎. 𝟏 𝐚𝐧𝐝 [𝑵𝑶] = 𝟎. 𝟎𝟏𝐌 In which direction will the reaction shift to reach equilibrium at 25oC? Why? (4) b.) Determine the equilibrium concentrations (show work on the back of this page). (10) c.) The equilibrium solution is placed in a sonicator, a device that passes sonic waves through solutions. This sonic disturbance causes 22% of the dissolved NO to bubble out of solution. Set up an ICE table and write out an expression to solve for the new equilibrium concentrations, but do not solve for x (use the back of this page). (6) 10. Dinitrogen monoxide decomposes to produce nitrogen gas and oxygen gas. Dr. Harris recently performed this reaction under controlled conditions, and generated the data shown below, which includes a table of time-dependent concentrations of the reactant, and a plot of ln[N2O] versus time which fits a linear trend. Answer the questions below. time, s [N2O] [N2] 0 3.000 1 2.582 2 2.222 3 1.913 4 1.646 5 1.417 [O2] ln [N2O] vs t 1.2 ln [N2O] 0.8 0.4 0 0 1 2 3 4 5 Time, s a.) Fill in the missing values in the table above. (10) b.) Write the rate law, including the value of k, with units. (5) c.) Find the initial rate of the reaction and the rate at t =8 sec (use back of page). (10) d.) BONUS: Find the half-life of the reaction. (3) BONUS 1. You have 100mL of a solution containing 0.25M methylamine, CH3NH2 (aq), and 0.2M methylammonium chloride, CH3NH3Cl. The Ka of the methylammonium ion is 2.17 x 10-11 M. Determine the change in pH of the solution following the addition of 4mL of 0.5M of hydrobromic acid. (5) 2. Use the table of initial rates to determine the rate law, including k, for the reaction: (5) 2𝑁𝑂2 (𝑔) + 𝑂3 (𝑔) ⇌ 𝑁2 𝑂5 (𝑔) + 𝑂2 (𝑔) [NO2] 0.65 1.10 1.70 [O3] 0.80 0.80 1.55 Rate (M/s) 2.61 x 104 4.40 x 104 1.32 x 105 3. If 51.5 mL of 0.1M HCl are mixed with 48.5mL of 0.1M NaOH, what is the final pH? (5) Equations and constants ΔGo = ΔHo -TΔSo ΔGo = -RT ln K (R = 8.314 J/mol K) ln (K2/K1) = (ΔH0/R)( 𝑇2 –𝑇1 𝑇1 𝑇2 ) 1st order: ln[𝐴]𝑡 = −𝑘𝑡 + ln[𝐴]0 K=𝑒 −∆𝐺𝑜 𝑅𝑇 ΔG = RT ln (Q/K) ln K = -ΔH/(RT) + ΔS/R 1 1 2nd order: = [𝐴] = 𝑘𝑡 + [𝐴] 𝑡 k = Ae-Ea/RT eln(x) = x pH = -log [H3O+] pHbuffer = pKa + log ([B]/[A]) 0 rate=k[A]m[B]n…… 10log(x) = x pH + pOH = 14 pKa = -log Ka