chemnotes1.15 / Microsoft Office Word Document
advertisement
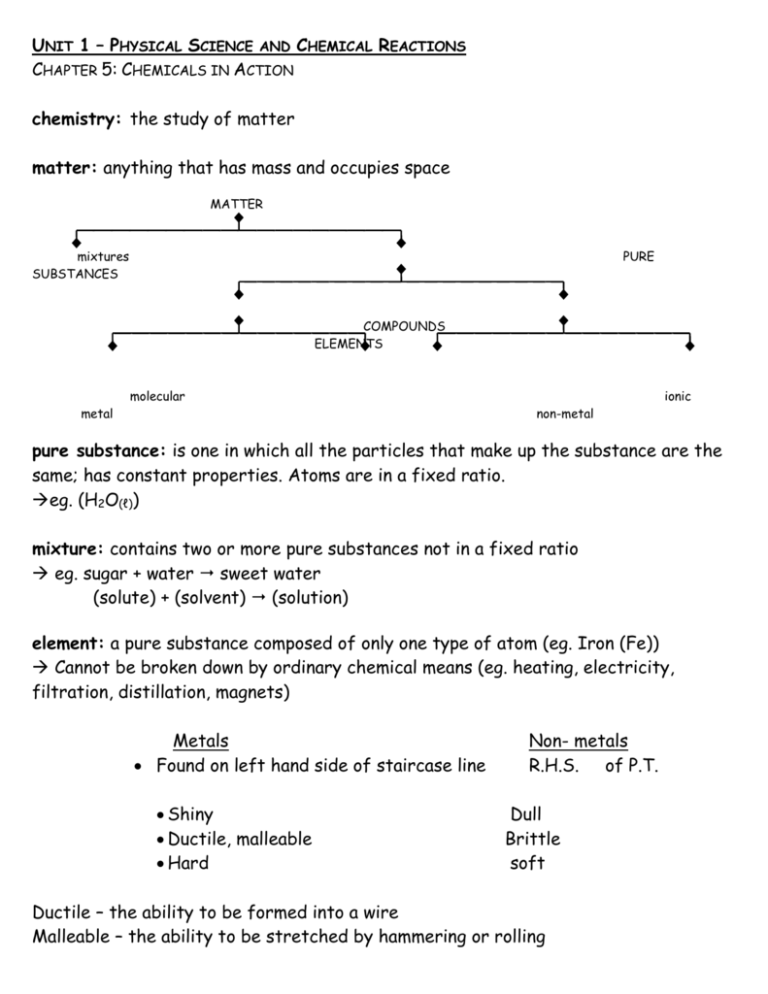
UNIT 1 – PHYSICAL SCIENCE AND CHEMICAL REACTIONS CHAPTER 5: CHEMICALS IN ACTION chemistry: the study of matter matter: anything that has mass and occupies space MATTER mixtures SUBSTANCES PURE COMPOUNDS ELEMENTS molecular metal ionic non-metal pure substance: is one in which all the particles that make up the substance are the same; has constant properties. Atoms are in a fixed ratio. eg. (H2O(ℓ)) mixture: contains two or more pure substances not in a fixed ratio eg. sugar + water sweet water (solute) + (solvent) (solution) element: a pure substance composed of only one type of atom (eg. Iron (Fe)) Cannot be broken down by ordinary chemical means (eg. heating, electricity, filtration, distillation, magnets) Metals Found on left hand side of staircase line Shiny Ductile, malleable Hard Non- metals R.H.S. of P.T. Dull Brittle soft Ductile – the ability to be formed into a wire Malleable – the ability to be stretched by hammering or rolling compound: pure substance composed of at least two types of elements (or atoms) eg. water (H2O(ℓ)); salt (NaCl(s)) Ionic Molecular Composed of 2 or more ions ( metal + non-metal) 2 or more non-metallic atoms Held together by an ionic bond covalent bond (e.g.) NaCl- sodium chloride CO2 – carbon dioxide The Modern Periodic Table Facts about hydrogen Group IA 1 valence electron Loses 1 electron to form a hydrogen ion Group VIIA 1 less electron than a Noble Gas A gas Can gain 1 electron to form a hydride ion Periodic Table Demitri Mendeleev created the first useable periodic table It became the most predictive device in all chemistry Elements are arranged in increasing order of Atomic Number (AN) The periodic table is divided into vertical columns (families/groups) and horizontal rows (periods) Atomic Number: (AN) the number of protons (p+) in the nucleus of an atom Group A Elements IA ~ Alkali Metals Soft, reactive IIA ~ Alkaline Earth Metals When compared to IA; harder or not as reactive IIIA ~ Aluminum Group IVA-VIA ~ Name according to first element in group VIIA ~ Halogens Very reactive VIIIA ~ Noble (inert) Gases All gases Non-reactive Group B Elements (Transition Elements) When compared to Group A elements; harder and more colourful, with higher boiling and melting points metalloids: located on both sides of the staircase line and have characteristics of metals and nonmetals Matter has a well defined underlying structure composed of the following: 1. Atoms The smallest whole part of an element that is still representative of the element A neutral particle composed of a nucleus containing protons (p+) and neutrons, and electrons (e-) outside the nucleus (the number of electrons equals the number of protons) 2. Ions A charged atom (The charge is caused by the transfer of an electron (or electrons) from one atom to another) The metallic atom (which loses an electron) has a positive charge and is called a cation - eg. Na+, Al3+ The non-metallic atom (which gains an electron) has a negative charge and is called an anion - eg. Clˉ, N3ˉ Other main features include: - Atomic Number = Number of protons - Number of protons = Number of electrons - An energy level represents a specific value of energy of an electron and corresponds to a general location - Period number = Number of energy levels occupied by electrons - The first three energy levels will have 2, 8, and 8 structures of electrons NOTE: 2, 8, and 8 should be written as follows started -8e-- -8e-- -8e-- -2e-- -2e-- -2e-- Each lower energy level must be filled to its maximum before the next level is - The electrons in the highest energy level are called valence electrons - Group number = Number of valence electrons (one exception is helium) (Group A only) - Max. number of electrons in each energy level = Max. number of atoms in each period Energy Level Diagrams For Atoms valence level: the outer-most energy level of an energy level diagram NOTE: It is important to include atom when writing the name ex. Lithium atom (Students will be expected to format all energy level diagrams for atoms as follows) Valence Electrons (Valence Level): - 1e-- 2e-Atomic Number: 3p+ Name: Lithium atom Symbol: Li Energy Level Diagrams For Ions ion: a charged atom Formed when a metal loses electrons to form a positive ion (cation) or when non-metals gain electrons to form a negative ion (anion) All atoms gain/lose electrons to become like the nearest noble gas (become stable) When naming ions: Metal (cation) names: stay the same Non-metal (anion) names: change (last three letters become “ide”) ex. Lithium ion (Students will be expected to format all energy level diagrams for ions as follows) - 2e-- Valence Electrons (Valence Level): 3p+ Atomic Number: Name: Lithium ion Symbol: Li+ ex. Fluoride ion (The atom Fluorine becomes the ion Fluoride) - 8e-- 2e-9p+ Valence Electrons (Valence Level): Atomic Number: Name: Fluoride ion Fˉ Symbol: Isotopes Atomic number = Number of protons in the nucleus of an atom Atomic mass (mass number) = Sum of the protons and neutrons in the nucleus of an atom isotopes: forms of an element that have the same number of protons but different numbers of neutrons Two Ways to Write Isotopes ex. Argon Atomic mass (AM) Atomic number (AN) ex. 40 18 Ar Atomic Mass argon-40 Atomic Mass Retrieving Information About Isotopes Atomic mass is given in both naming forms Atomic number is either given or must be looked up on the Periodic Table Number of protons = Atomic number Number of electrons = Number of protons Number of neutrons = Atomic mass minus atomic number (n = AM – AN)








