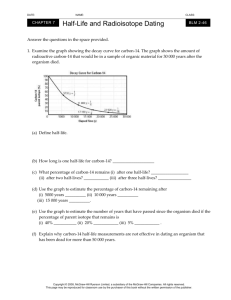
Unit 9 Optional Review: Page 386 1-17, 23, 24, 28, 29 Carbon film
advertisement

Unit 9 Optional Review: Page 386 1-17, 23, 24, 28, 29 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Carbon film Law of superposition Uniformitarianism Unconformity Half life Index fossil Absolute ages Permineralized remains Cast B C B D D C D D 23. Angular unconformities have tilted layers on the bottom, erosion and then parallel layers. Disconformities have parallel layers of sedimentary rock on the bottom, erosion and then parallel layers of sedimentary rock on top. Non-conformities have igneous or metamorphic rocks on the bottom, erosion, and then parallel layers of sedimentary rock on top. 24. Relative age is a comparison of the age of one rock to another. Also can tell you what comes first. Absolute age gives a number with a unit of time. Rocks that are aged absolutely (using radiometric dating) can be used to help determine better estimates for the relative ages of rocks. 28. 3 29. Bottom left graph. Page 418 2, 3, 9, 10, 12, 13, 14, 16, 27 2. geologic time scale 3. eon 9. A 10. b 12. A 13. c 14. b 16. Too much harmful UV radiation from the sun reached Earth’s surface 27. A- Mesozoic era; B- Precambrian time; C- Cenozoic Era; D- Paleozoic Era REMEMBER YOU NEED TO KNOW THE EONS, ERAS AND PERIODS IN ORDER. YOU ALSO NEED TO KNOW THE ERA AND PERIOD OF THE 15 EVENTS. Half Life Review 1. If the half-life of iodine-131 is 8.10 days, how long will it take a 50.00 g sample to decay to 6.25 g? 24.3 days 2. The half-life of hafnium-156 is 0.025 s. How long will it take a 560 g sample to decay to onefourth its original mass? .05 seconds 3. Chromium-48 has a short half-life of 21.6 h. How long will it take 360.00 g of chromium-48 to decay to 16.25 g 86.4 hr 4. Potassium-42 has a half-life of 12.4 hours. How much of an 848 g sample of potassium-42 will be left after 62.0 hours? 53 g 5. What percentage of a radioactive element will be left after: a. 1 half-life _50_____ b. 2 half-lives __25___ c. 3 half-lives ____12.5_ 6. How many half-lives have passed for each of the following samples: a. 50% of the original radioactive material remains b. 25% of the original radioactive sample remains c. 12.5% of the original radioactive sample remains ____1_______ ____2________ ___3_________ 7. If a rock sample originally contained 12 g of Uranium-235, how much will be left after: a. 1 half-life __6_____ b. 2 half-lives ___3___ c. 3 half-lives __1.5__ 8. Uranium-235 has a half-life of 700 million years. How much of the 12 g sample of Uranium-235 will be left after : a. 700 million years ___6____ b. 1400 million years ___3____ 9. Carbon-14 is a radioactive element that decays into Notrogen-14. The half-life of Carbon-14 is 5730 years. What percentage of Carbon-14 and Nitrogen-14 will be left in a dinosaur bone after: 5730 years: % of Carbon-14 ___50______ % of Nitrogen-14 ___50_____ 11,580 years: % of Carbon-14 __25_______ % of Nitrogen-14 ___75_____ 17,310 years: % of Carbon-14 __12.5_______ % of Nitrogen-14 __87.5______ 2. A is younger than X 3. 5 is an angular unconformity 3. A is younger than the fault. The fault does not disturb A. Order of Events- B, C, D, E, F, G, H, X, Y, A, Z