Mixtures and Solutions
advertisement

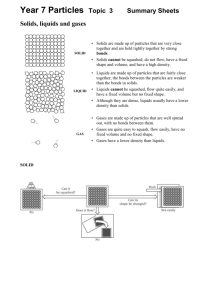
Solutions/Mixtures Notes Mixture: a combination of two or more substances that are not chemically combined Types of mixtures Heterogeneous Mixtures The amount of each substance in different samples of a heterogeneous mixture varies. Example(s): A suspension is a mixture in which large particles of a material are more or less evenly dispersed throughout a liquid or gas. Example(s): Particles in a suspension may settle over time, and may be filtered out Some combinations of liquids will not mix, but will separate spontaneously. Example(s): Liquids that do not mix with each other are immiscible. One way to separate two immiscible liquids is to carefully pour the less dense liquid off the top. This is called decanting. A colloid is a mixture consisting of tiny particles that are intermediate in size between those in solutions and those in suspensions and that are suspended in a liquid, solid or gas. Particles in a colloid are too small to settle out However, particles in a colloid are large enough to scatter light that passes through: this is called the Tyndall effect. Examples of familiar materials that are colloids include gelatin desserts, egg whites, and blood plasma Somme immiscible liquids can form colloids An emulsion is any mixture of two or more immiscible liquids in which one liquid is dispersed in the other. Homogeneous Mixtures Homogeneous mxitures not only look uniform, but are uniform. Example(s): A solution is a homogeneous mixture of two or more substances uniformly dispersed throughout a single phase. In a solution, the solute is the substance that dissolves in the solvent. The solvent is the substance in which the solute dissolves. Miscible liquids mix to form solutions. One way to separate miscible liquids is by distillation, which works when the two miscible liquids have different boiling points Water is a common solvent, but some liquid solutions contain no water. Example(s): Other states of matter can also form solutions The air you breathe is a solution of nitrogen, oxygen, argon, and other gases. The liquid element mercury dissolves in solid silver to form a solution called an amalgam, which can be used to fill cavities in teeth. An alloy is a solid or liquid mixture of two or more metals.