[OH - ] < 1.0 x 10
![[OH - ] < 1.0 x 10](http://s3.studylib.net/store/data/007110510_1-bfa16e7ddc01a3c98cff3c549f0ddc6b-768x994.png)
Name ____________________________________________ Date _________________________ Period__
Sample Problems for Chapter 14: Acids and Bases
14.5 Ionization of Water
(Read pgs. 455-458 in the chemistry textbook)
1. (a) What does amphoteric mean?
(b)
TRUE / FALSE
Water is amphoteric.
(circle one)
2. (a) Write the chemical equation for the
ionization of water
as it reacts with itself to form both
an acid and a base.
(b) How is this reaction commonly "shortened"?
3. (a) In PURE WATER, the what is the concentration of hydronium ion, [H
3
O
+
]?
(b) In PURE WATER, what is the concentration of hydroxide ion, [OH
-
]?
(c) What is the meaning of the square brackets around the symbols?
1
4. (a) If
[OH ] = [H + ]
the solution is ________________________________
(b) If
[OH
-
] > [H
+
]
the solution is ________________________________
(b) If
[H
+
] > [OH
-
]
the solution is ________________________________
5. (a) If
[OH ] = 1.0 x 10 -7 M
the solution is ________________________________
(b) If
[OH ] > 1.0 x 10 -7 M
the solution is ________________________________
(b) If
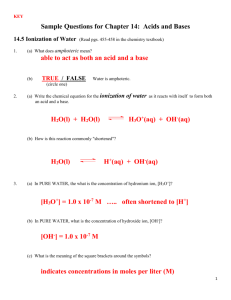
[OH ] < 1.0 x 10 -7 M
the solution is ________________________________
6. (a) If
[H + ] = 1.0 x 10 -7 M
the solution is ________________________________
(b) If
[H + ] > 1.0 x 10 -7 M
the solution is ________________________________
(b) If
[H + ] < 1.0 x 10 -7 M
the solution is ________________________________
7. If [H
+
] = 1.0 x 10
-3
M, is the solution acidic, basic, or neutral? Explain.
8. If [H
+
] = 1.0 x 10
-9
M, is the solution acidic, basic, or neutral? Explain.
9. If [OH ] = 1.0 x 10 -9 M, is the solution acidic, basic, or neutral? Explain.
10. If [OH
-
] = 1.0 x 10
-3
M, is the solution acidic, basic, or neutral? Explain.
2
11. Indicate whether each of the following solutions are acidic, basic, or neutral.
(a) [H + ] = 2.0 x 10 -5 M
(b) [H
+
] = 1.4 x 10
-9
M
(c) [OH
-
] = 8.0 x 10
-3
M
(d) [OH
-
] = 3.5 x 10
-10
M
(e) [H
+
] = 6.0 x 10
-12
M
(f) [H
+
] = 1.4 x 10
-4
M
(g) [OH
-
] = 5.0 x 10
-12
M
(h) [OH
-
] = 4.5 x 10
-2
M
3
12. (a) What is the
ion product constant
of water?
(b) What is the symbol of the ion product constant for water? ________________________________
(c ) What units are used for the ion product constant of water?
________________________________
(d) Calculate the ion product constant for pure water (neutral).
13. (b) If NaOH(s) is added to pure water
[H + ] / [OH ] increases.
(circle one)
(b) If HCl (aq) is added to pure water
[H + ] / [OH
(circle one)
] increases.
14. (a) If H
2
CO
3
(aq) is added to pure water
[H + ] / [OH ] increases and the solution will
(circle one)
be
ACIDIC / BASIC.
(circle one)
(b) ) If NH
3
(aq) is added to pure water
[H + ] / [OH ] increases and the solution
(circle one)
will be
ACIDIC / BASIC.
(circle one)
15. What is the equation to be used when [H
+
] or [OH
-
] need to be calculated for ANY solution?
4
16. (a) A vinegar solution has [H
+
] = 2.0 x 10
-3
M. Calculate the [OH
-
] of the vinegar solution?
(b) Is this solution acidic, basic, or neutral? Explain.
17. (a) What is the [H
+
] of an ammonia cleaning solution with an [OH
-
] = 4.0 x 10
-4
M?
(b) Is this solution acidic, basic, or neutral? Explain.
18. Calculate the [H
+
] of each aqueous solution with the following [OH
-
].
(a) Coffee, 1.0 x 10
-9
M
5
18. Continued:
(b) Soap, 1.0 x 10 -6 M
(c) Cleanser, 2.0 x 10 -5 M
(d) Lemon juice, 4.0 x 10 -13 M
19. Calculate the [H
+
] of each aqueous solution with the following [OH
-
].
(a) NaOH, 1.0 x 10
-2
M
6
19. Continued:
(b) aspirin, 1.8 x 10
-11
M
(c) milk of magnesia, 1.0 x 10
-5
M
(d) seawater, 2.0 x 10
-6
M
20. Calculate the [OH
-
] of each aqueous solution with the following [H
+
].
(a) vinegar, 1.0 x 10 -3 M
7
20. Continued:
(b) urine, 5.0 x 10 -6 M
(c) ammonia, 1.8 x 10
-12
M
(d) NaOH, 4.0 x 10 -13 M
8