Expression and Purification Problem Set
advertisement

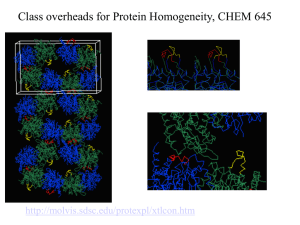
Biochemistry Lab Expression and Purification Homework Assignment Name: Instructions: This homework assignment is open notes, book, and internet. We expect you to research your answers and to use YOUR voice/words; the assignment is not group work. Please do not work together or discuss aspects of the assignment with others in the course. Part of how you will learn the material is by looking up and answering these questions. Turn the answers in TYPED ONLY. Submit final answer in class. Emailed assignments will NOT be accepted. Remember: We want more than simple declarative statements. Be specific and biochemical in your answers. Reasoning, biochemical details, and examples supporting your work will earn you maximum points. Simple, short answers will result in less points. These are essay problems; bulleted or outlined answers will not be accepted. Drawings or images are only to be used in support and should not be the main feature of your answer(s). Reference material. Feel free to investigate and find other resources for your answers. 1) You have a wonderful new clone (Toreroase) that may solve all of the world’s hunger problems by converting paper (cellulose) into monosaccharides. You need to make a large amount of the protein. What considerations do you need to make when planning the production of Toreroase? Be specific and thorough. Why would you pick a particular host/expression system? What detection method(s) will you use to detect it? (Hint: think of the kind of reaction the enzyme catalyzes.) You have all of the tools and vectors or plasmids you can buy or make. 2) What are the pros and cons of inclusion bodies? What causes inclusion bodies to be formed? 3) Explain what a fusion protein is and what affinity chromatography is. Outline the pros and cons of each of the various affinity tags. What are the differences in the fusion partners? What impact might the fusion partner have on a protein? What can be done about the fusion partner in the final protein product? What are the different mechanisms/biochemistry of binding and elution for the fusion proteins? 4) What is the difference between the pQE, the pET, and the pGEX plasmids? Your answer should include more than the kind of fusion protein. In which case would you choose a strain containing pLysE and/or DE3 for protein expression? 5) What are the key differences between various strains of bacteria for protein expression? Why would you use one over another? Why not just use DH5alpha cells to express and make protein? 6) Create a simple outline (yes, here you can use bullets and outlines!) starting from plasmid DNA with your clone in it (pick one, any one), transform competent cells (choose the cells, describe competent cells and transformation process), and how you would go all the way through from lysis to purification of a GST-tagged protein. 7) In class we briefly discussed immunoprecipitation/pull-down assays. Describe these assays and the kinds of experiments in which the assays are used. Find and describe a protocol for a pull-down/immunoprecipitation of an affinity or epitope tagged fusion protein. 8) Why would you choose to use bacteria vs yeast, mammalian, or insect cells to express a recombinant protein? 9) Your protein just does not express well in BL21 cells. No inclusion bodies, just very low expression levels. Provide a plan to troubleshoot and overcome this problem. 10) Describe the common methods of lysing bacterial cells for protein expression. How do these methods differ from the technique we are using in our class? Be Neat, Be Precise, Be Complete! Avoid pronouns such as it, that, those and them, to ensure that you are clear and communicate your ideas well.