File - Mrs. LeCompte
advertisement

5-2: Passive Transport
PASSIVE TRANSPORT
Concentration Gradient = regular, graded concentration change over a distance in a particular
direction
Passive Transport = diffusion of a substance across a biological membrane
Moves particles down their concentration gradient (that is, from [Hi] to [Lo])
Does NOT require cellular energy
Is regulated by the permeability of the membrane
Net Directional Movement = overall movement away from the center of concentration, which
results from kinetic molecular movement in all directions
Diffusion = the net movement of a substance down a concentration gradient
Results from the intrinsic kinetic energy of molecules
Result from random molecular motion even though net movement may appear directional
Continues until dynamic equilibrium is reached = the molecules continue to move, but
there is NO net directional movement
A substance diffuses down its own concentration gradient and is not affected by the
gradients of other substances
Differs from bulk flow = when all the molecules move in the same direction at the same
time
o Ex. waterfalls or blood in the body
Water diffuses freely across membranes
Osmosis = diffusion of water down its gradient
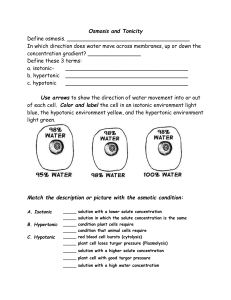
Hypertonic Solution = the solution with a higher solute concentration
Hypotonic Solution = the solution with a lower solute concentration
Isotonic Solution = solution with an equal solute concentration
o If two solutions are isotonic, no net movement of water is perceived
Water Potential (ψ)
o is often used to predict the movement of water between two areas of differing
concentrations.
o Water Potential = Pressure Potential + Solute Potential
ψ
=
For open systems, ψp = 0
Ψs = - iCRT
Where:
ψp
+
ψs
o i = ionization constant, C = concentration, R = pressure constant, and T =
temperature in K
So the equation for open systems becomes: ψ = ψs = - iCRT
For pure water, the ψ = 0
Once solutes are added, the solution becomes more concentrated (ψ lowers). {more -}
Water always moves from areas of HIGH ψ to LOW ψ. (That is, from areas of high
water to areas of low water OR from less concentrated areas to more concentrated areas.)
Water and Cells
Animal Cells lack a cell wall.
In isotonic environments, animal cells remain of concentrated volume.
In hypertonic environments, animal cells lose water by osmosis and shrivel = crenate
In hypotonic environments, animal cells gain water by osmosis and swell
o Danger: could perhaps lyse (burst)
Prevent excessive water loss or uptake by:
o Living in isotonic environments (ex. Marine invertebrates make “compatible”
solutes inside their cells)
o Osmoregulation = removing water in hypotonic environments and retaining
water/pumping out salts in hypertonic environments
Organisms with Cell Walls (Plants, Fungi, Prokaryotes, and some Protists)
In a hypotonic environment, water moves into plant cell until the internal pressure against
the cell wall will not allow more to enter
o Turgor = firmness or tension in walled cells that are in hypotonic environments
o Ideal state for most plant cells
o Provides mechanical support for plants (helps them stand up)
o Requires cells to be hypertonic to their environment.
In isotonic environments, turgor is lost and they become flaccid (limp) wilt
In hypertonic environments, they lose water by osmosis and plant cells may plasmolyze.
o Plasmolysis = phenomenon where the loss of water causes the plasma membrane
to pull away from the cell wall
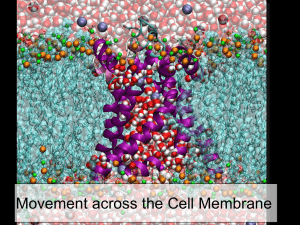
Facilitated Diffusion = diffusion of (large) molecules and ions across a membrane with the aid
of transport proteins
Is a form of passive transport
Have a specific binding site
Can become saturated or inhibited
May cause a conformational change that translocates the binding site and the attached
solute
May provide a selective channel across a membrane
Gated Channels = protein channels that open in response to an impulse or chemical
stimulus