File
advertisement

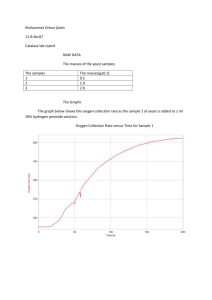
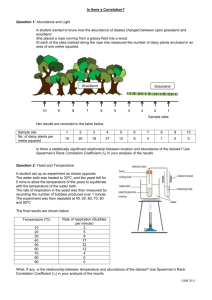
Name: ____________________________________ Date: ____________________________ Period: ___________ Chemical Reactions Lab—Part 1 All students Questions: What are the signs of a chemical reaction? Do all reactions form heat (exothermic)? What is the role of a catalyst in a reaction? Research: definitions are on the attached page. Add them to the lab. Exothermic reaction: _______________________________________________________________________________ ________________________________________________________________________________________________ Endothermic reaction:_______________________________________________________________________________ _________________________________________________________________________________________________ Catalyst:__________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Signs of a chemical change: _________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ Today we will be making elephant toothpaste using hydrogen peroxide and a yeast solution, to determine the signs of a chemical reaction. The reaction is below. Identify and count the elements in the reaction. 2H2O2 --› 2H2O + O2 Is the reaction balanced? _____________________________________________________________________________ __________________________________________________________________________________________________. Hypothesis: We will be making the toothpaste using yeast or a yeast solution. Which do you think will create the bigger reaction, the substance made with yeast or with the yeast solution? I think ___________________________________________________________________________________ will make a larger reaction. NOTE: Based on the materials needed, some groups may be doing the experiment using a yeast solution and some may be doing the experiment with dry yeast. STOP—Make a prediction above, before moving on. Will the plain yeast or the yeast solution (yeast & water mixture) make a larger reaction? Chemical Reactions Lab—Part 2, alternate lab Directions: Read over the procedures and the observations for Trial 1 and Trial 2. You will need to do this in order to complete the back of this sheet. Trial 1 1. Students made observation on the properties of yeast and peroxide (H2O2). 2. Students placed peroxide and soap in a drink bottle. 3. Yeast was added to the peroxide mixture. 4. Observations were made on the reaction. You will find observations in the data table for trial 1. Trial 2 1. Students added warm water to the yeast. 2. Students made observation on the properties of the yeast mixture and peroxide (H2O2). 3. Students placed peroxide and soap in a drink bottle. 4. The yeast mixture was added to the peroxide mixture. 5. Observations were made on the reaction. You will find observations in the data table for trial 2. DATA Trial 1 observations— Properties of the yeast Properties of H2O2 Tan Grainy Slight smell-sour Looks like sand Solid Small pieces cool Clear Liquid Slight smell Cool—cooler than yeast Observations from lab Lots of foam—airy, lots of air bubble, whitish Foam is very warm, bottom of bottle is hot Foam covers half the tray Trial 2 observations— Properties of the yeast mixture Tan Thick Sour smell warm Properties of H2O2 Clear Liquid Slight smell Cool—cooler than yeast Observations from lab Very hot—foam and bottle, hotter than trial 1 Lots of foam—covers bottom of container, more than in trial 1 Foam is creamier, few air bubbles Conclusions: DIRECTIONS: Use part 1 and part 2 and the data given to answer the questions below. 1. What type of data was gathered—qualitative or quantitative? Explain your answer. _______________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ 2. What was your hypothesis? ____________________________________________________________________ ___________________________________________________________________________________________ 3. What was the independent variable in the experiment? _____________________________________________ ___________________________________________________________________________________________ 4. What was the dependent variable in the experiment? _______________________________________________ ___________________________________________________________________________________________ 5. What was the experimental group? ______________________________________________________________ ___________________________________________________________________________________________ 6. What was the control group? ___________________________________________________________________ ___________________________________________________________________________________________ Conclusion paragraph: Restate your hypothesis. Clearly state if the hypothesis was correct or incorrect. Compare the data from Trial 1 and Trial 2 to defend your conclusion that the hypothesis was correct or incorrect. __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ Vocabulary Exothermic reaction- a reaction in which energy (heat or light) is produced as part of the product Endothermic reaction-a reaction in which energy (heat or light) is used to start the reaction Catalyst-a substance that is used to start a reaction, but does not change as part of the reaction Signs of a chemical reaction: temperature change (hot or cold), light formed, new substance formed odor change, color change