CHEM242_April2014 - Heartland Community College
advertisement

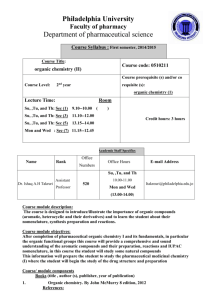
Heartland Community College Master Course Syllabus Division Name: MS Division Course Prefix and Number: CHEM 242 Course Title: Organic Chemistry II DATE PREPARED: December 15, 1999 DATE REVISED: February 16, 2012 PCS/CIP CODE: 11 400504 IAI NO. (if available): Major Code CHM 914 EFFECTIVE DATE OF FIRST CLASS: Spring 2012 CREDIT HOURS: 5 CONTACT HOURS: 7 LECTURE HOURS: 4 LABORATORY HOURS: 3 CATALOG DESCRIPTION: Prerequisite: CHEM 241 or equivalent. This course is a continuation of Organic Chemistry I. This course will focus on the synthesis, reactivities, and mechanisms of various organic reactions. Topics will include the study of aldehydes, ketones, carboxylic acids, esters, amines, amides, aromatic derivatives, and biologically important molecules. One three hour lab each week will emphasize the synthesis, characterization, and identification of organic compounds that feature different functional groups. TEXTBOOKS such as: Carey, Francis A. and Robert M. Guiliano. Organic Chemistry. 8th Edition. New York, NY: McGraw Hill, 2010. Print. Solomons, T.W. Graham and Craig B. Fryhle. Organic Chemistry. 9th Edition. Hoboken, NJ: John Wiley & Sons, 2007. Print. Wade, L.G. Organic Chemistry. 8th Edition. Upper Saddle River, NJ: Prentice Hall, 2012. Print. A comparable text that addresses at a minimum the topics listed in the Course Outline and that provides students with the opportunity to achieve the learning outcomes for this course may be substituted. RELATIONSHIP TO ACADEMIC DEVELOPMENT PROGRAMS AND TRANSFERABILITY: CHEM 242 fulfills 5 semester hours of elective credit for the A.A., A.S. or A.A.S. degrees. It should transfer to most colleges and universities as an elective course. However, since it is not part of the General Education Core Curriculum described in the Illinois Articulation Initiative, students should check with an academic advisor for information about its transferability to other institutions. This course should articulate as the equivalent of an IAI baccalaureate major course; refer to the IAI web page for further information at www.itransfer.org. LEARNING OUTCOMES: This is the second course of a two-semester sequence intended for those students with an interest in a science major, such as chemistry, chemical engineering, biology, or physics, or a pre-professional major, such as pre-medical, pre-dental, or pre-pharmacy. This course covers the detailed mechanistic explanations of a variety of synthetic organic reactions, as well as the prediction of reagents necessary to bring about a series of synthetic transformations. These skills will ultimately be used in the planning of the total synthesis of a diverse group of organic compounds. COURSE OUTCOMES: By the end of this course, students will have developed an understanding of the fundamental concepts of organic chemistry, as well as critical thinking and analysis skills. This will be achieved by 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. applying concepts of stability and reactivity to conjugated and aromatic systems, determining the chemical reactivity of aromatic molecules with both electrophiles and nucleophiles, understanding the oxidative and reductive reactivity of alcohols, aldehydes, ketones, and carboxylic acids, reviewing and expanding upon the reactivity of alcohols, ethers, and analogous sulfur-containing compounds, understanding the properties of a variety of carbonyl compounds, including aldehydes, ketones, carboxylic acids, esters, anhydrides, and acyl halides, determining the chemical reactivity of these classes of carbonyl compounds, involving wide range of nucleophiles and electrophiles, understanding the methods for preparing carbon-based nucleophiles, such as organolithium and Grignard reagents, then reacting these nucleophiles with a variety of electrophiles, understanding the methods for preparing and reacting enols and enolates, understanding the properties and nucleophilic reactivity of amines, as well as their preparation using reductive and substitution reactions, and applying these reactions to biologically-relevant molecules, like carbohydrates and amino acids. GENERAL EDUCATION OUTCOMES: PS1, PS2, PS3, PS4, CT1, CT2, CT3, CO1 RANGE OF ASSESSMENT METHODS: Problem sets, quizzes, exams, laboratory reports, technique (practical) assessments COURSE/LAB OUTLINE: Lecture topics - Conjugated systems and their reactivity - Aromatic systems (nomenclature and reactivity) - Alcohols, ethers, and sulfur-analogs (nomenclature and reactivity) - Aldehydes and ketones (nomenclature and reactivity) - Organometallic compounds - Carboxylic acids and their derivatives (nomenclature and reactivity) - Amines (nomenclature and reactivity) - Applications in carbohydrate, lipid, and amino acid chemistry - Total synthesis Laboratory experiments - Diels-Alder Cycloaddition - Electrophilic Aromatic Substitution: Bromination - Electrophilic Aromatic Substitution: Friedel-Crafts Acylation - Green Oxidation of a Secondary Alcohol - Reduction and Acetal Protection - Grignard Generation and Nucleophilic Attack - Esterification: Synthesis of Aspirin and Wintergreen from a Common Precursor - Aldol Condensations METHOD OF EVALUATION (Tests/Exams, Grading System): Problem Sets: 10 – 15 % Quizzes: 10 – 15 % Exams: 40 – 50 % Laboratory work: 20 – 40 % Grades will be based on a set scale: A: 85.0% B: 75.0 – 84.9% C: 65.0 – 74.9% D: 60.0 – 64.9% F: ≤ 59.9% An incomplete grade may be given to a student who, by the withdrawal date, can reasonably be expected to pass the course. Incompletes may be granted only when justified by extreme circumstances (e.g., serious illness, accident, death or serious illness in the immediate family). Incomplete grades are not given for such reasons as unjustified failure to appear for the final examination. A written agreement, outlining the requirements to be met, must be signed by the instructor and the student. The agreed-upon requirements must be completed no later than the end of the following semester (spring semester for incompletes granted during the fall, and the following fall for incompletes given during the spring and summer semesters). By the agreed-upon date, the instructor will assign a grade or the incomplete will be changed to an F if the requirements are not completed. REQUIRED WRITING AND READING: This course will require the reading of approximately 600 pages of text from both the lecture and lab textbooks over a 16-week period. Written analysis will be required in the form of problem sets, quizzes, exams, and both formal and informal laboratory reports.