Experiment 5: Iodimetric Titration of Vitamin C
advertisement

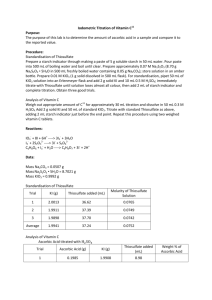
Experiment 5: Iodimetric Titration of Vitamin C11 Purpose: To determine how much, if any ascorbic acid (vitamin C) is actually found in supplemental sources of vitamin C. Procedure: Part 1: Standardization of Thiosulfate Solution 1. Group part: prepare starch indicator solution: a. 5g soluble starch paste in 50mL water. Pour into 500mL boiling water and boil until clear. 2. Prepare about 0.07M Na2S2O3 by dissolving 8.70g Na2S2O3*5H2O in 500mL boiling water containing 0.05g Na2CO3 a. Prepare approximately 0.01M KIO3 by dissolving 1.0g solid reagent in a 500mL flask 3. Standardize: pipet 50mL KIO3 into an Erlenmeyer flask a. Add 2.0g KI(s) and 10mL of 0.5M H2SO4 b. Titrate with thiolsulfate until color is gone c. Repeat titration until three good trials Part 2: Analysis of Vitamin C 1. Weigh amount ascorbic acid for a 30mL titration a. Dissolve in 50mL 0.3M HCl 2. Add 2g KI (s) and 50mL standard KIO3 a. Titrate with standard thiosulfate and add 2mL starch indicator before endpoint 3. Repeat using two vitamin C tablets a. Determine the weight percent of vitamin C, standard deviation, and accuracy b. Three good trials Reactions: 𝐼𝑂3− + 8𝐼 + 6𝐻 + ↔ 3𝐼 − + 3𝐻2 𝑂 𝐼3− + 2𝑆2 𝑂3− ↔ 3𝐼 − + 𝑆4 𝑂62− 𝐶6 𝐻6 𝑂6 + 𝐼3− + 𝐻2 𝑂 ↔ 𝐶6 𝐻8 𝑂7 + 3𝐼 − + 2𝐻 + Data: Part 1 Mass Na2CO3=0.0522g Mass Na2S2O7*5H2O=8.7278g Mass KIO3(s) =1.0015g Trial Mass KI Thiosulfate added Molarity Thiosulfate 1 2 3 Average 2.0088g 2.0041g 2.0014g 2.0048g 38.0mL 37.3mL 37.4mL 37.7mL 0.0472M 0.0756M 0.0754M 0.0751M Part 2: Ascorbic acid treated with H2SO4 Trial 1 Mass KI 1.9999g Mass Ascorbic Acid 2.005g Thiosulfate added 9.3mL Wt % A.A. 90.5% Amount Vitamin C 0.3004g 0.3122g 0.3063g Thiosulfate added 3.8mL 2.5mL 3.15mL Wt% vitamin C 74.30% 74.25% 74.28% KI titrated with Thiosulfate Trial 2 3 Average Amount KI 2.0011g 2.0002g 2.00065g Calculations: Mass ascorbic acid needed: 0.0759𝑚𝑜𝑙 𝑆2 𝑂3 ∗ 0.03𝐿 1𝑚𝑜𝑙 𝐶6 𝐻6 𝑂6 176.1259𝑔 ∗ ∗ = 0.2005𝑔 𝐶6 𝐻6 𝑂6 2𝑚𝑜𝑙𝑆2 𝑂3 1𝑚𝑜𝑙 𝐼3− 1𝑚𝑜𝑙 Ascorbic Acid 1𝑚𝑜𝑙 1 ∗ = 0.0094𝑀 𝐾𝐼𝑂3 214𝑔 𝐾𝐼𝑂3 0.5𝐿 3𝑚𝑜𝑙 𝐼 − 1𝑚𝑜𝑙 𝑆2 𝑂3 0.0094𝑀 𝐾𝐼𝑂3 ∗ 0.005𝐿 ∗ ∗ = 0.00282𝑚𝑜𝑙𝑆2 𝑂3 1𝑚𝑜𝑙 𝐼𝑂3 1𝑚𝑜𝑙 𝐼3− 0.00282𝑚𝑜𝑙 𝑆2 𝑂3 = 0.0742𝑀𝑆2 𝑂3 0.038𝐿 1.0015𝑔 𝐾𝐼𝑂3 ∗ Mol 𝐈𝟑− 1𝑚𝑜𝑙 𝑆2 𝑂3 1𝑚𝑜𝑙 𝐼3 ∗ = 3.49𝑥10−4 𝑚𝑜𝑙 𝐼3− 𝑒𝑥𝑐𝑒𝑠𝑠 1𝑚𝑜𝑙 𝑁𝑎2 𝑆2 𝑂3 2𝑚𝑜𝑙 𝑆2 𝑂3 3𝑚𝑜𝑙 𝐼3− (0.0094𝑀)(0.05𝐿) = 4.7𝑥10−4 𝑚𝑜𝑙 𝐾𝐼𝑂3 ∗ = 0.00141𝑚𝑜𝑙 𝐼3− 1𝑚𝑜𝑙 𝐾𝐼𝑂3 (0.0093𝐿 𝑆2 𝑂3 ) ∗ (0.0751𝑀 𝑆2 𝑂3 ) ∗ 0.00141𝑚𝑜𝑙 − 3.49𝑥10−4 𝑚𝑜𝑙 𝑒𝑥𝑐𝑒𝑠𝑠 = 0.001031 𝑚𝑜𝑙 𝐼3− Grams Ascorbic Acid: 0.001031 𝑚𝑜𝑙 𝐼3− ∗ 1 𝑚𝑜𝑙 𝐴. 𝐴. 176.1256𝑔 ∗ = 0.1816𝑔 𝐴. 𝐴. 1 𝑚𝑜𝑙 𝐼3− 1 𝑚𝑜𝑙 𝐴. 𝐴. Weight percent of Ascorbic Acid: 𝑔 𝑐𝑎𝑙𝑐𝑢𝑙𝑎𝑡𝑒𝑑 0.1816𝑔 = 𝑥100 = 90.5% 𝑔 𝑠𝑡𝑎𝑟𝑡𝑒𝑑 𝑤𝑖𝑡ℎ 0.2005𝑔 Weight percent of Vitamin C: 0.0038𝐿𝑆2 𝑂3 ∗ 0.0751𝑀𝑆2 𝑂3 ∗ 1𝑚𝑜𝑙 𝑆2 𝑂3 1𝑚𝑜𝑙 𝐼3 ∗ = 0.000143𝑚𝑜𝑙 𝑒𝑥𝑐𝑒𝑠𝑠 𝐼3− 1𝑚𝑜𝑙 𝑁𝑎2 𝑆2 𝑂3 2𝑚𝑜𝑙 𝑆2 𝑂3 (0.0094𝑀)(0.05𝐿) = 4.7𝑥10−4 𝑚𝑜𝑙 𝐾𝐼𝑂3 ∗ 3𝑚𝑜𝑙 𝐼3− = 0.00141𝑚𝑜𝑙 𝐼3− 1𝑚𝑜𝑙 𝐾𝐼𝑂3 0.00141𝑚𝑜𝑙 𝐼3− − 0.000143𝑚𝑜𝑙 𝑒𝑥𝑐𝑒𝑠𝑠 𝐼3− = 0.001267𝑚𝑜𝑙 𝐼3− Grams Vitamin C calculated: 0.001267 𝑚𝑜𝑙 𝐼3− ∗ 1 𝑚𝑜𝑙 𝐴. 𝐴. 176.1256𝑔 ∗ = 0.2232𝑔 𝑉𝑖𝑡𝑎𝑚𝑖𝑛 𝐶 1 𝑚𝑜𝑙 𝐼3− 1 𝑚𝑜𝑙 𝐴. 𝐴. Percent yield Vitamin C 0.2232𝑔 ∗ 100% = 74.30% 0.3004𝑔 Weight percent claimed by manufacturer: 0.500𝑔 𝑉𝑖𝑡𝑎𝑚𝑖𝑛 𝐶 ∗ 100% = 81.62% 0.6126𝑔 𝑡𝑎𝑏𝑙𝑒𝑡 Standard Deviation: 2 2 2 √∑(𝑥𝑖 − 𝑥𝑎𝑣𝑔 ) = √(74.30 − 74.28) + (74.25 − 74.28) = 0.036 𝑛−1 1 Questions: 1. Equations: 𝐼𝑂3− + 8𝐼 + 6𝐻 + ↔ 3𝐼 − + 3𝐻2 𝑂 𝐼3− + 2𝑆2 𝑂3− ↔ 3𝐼 − + 𝑆4 𝑂62− 𝐶6 𝐻6 𝑂6 + 𝐼3− + 𝐻2 𝑂 ↔ 𝐶6 𝐻8 𝑂7 + 3𝐼 − + 2𝐻 + 2. The undissolved solid from the tablets were fillers and additives which may include calcium carbonate 3. The amount of vitamin C in the tablet was close to the manufacturers claim. The average percent Vitamin C was 74.28% and the claimed percent was 81.62%. Conclusion: The goal of this lab was to determine how much Vitamin C was in a Vitamin C tablet and compare it to the manufactures claim. This was done by titrating different samples with Thiosulfate. Overall, the percent Vitamin C recovered was close to the claimed amount. The average percent Vitamin C was 74.28% and the claimed percent was 81.62%. The standard deviation was relatively low at only 0.036. Error from this lab may have come from not dissolving the tablet all the way before titrating it with the Thiosulfate.