Yeast-Enzyme Concentration
advertisement

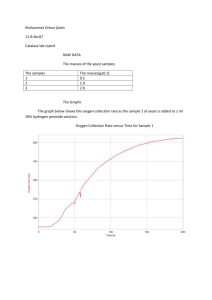
Ozanalp Eryılmaz 11/B EXPERIMENT: THE EFFECT OF ENZYME CONCENTRATION ON THE ACTIVITY OF CATALASE DESIGN Aim To investigate the relationship between the enzyme concentration and the activity of catalase. Research Question How does the rate of oxygen production by the decomposition of hydrogen peroxide vary with the increasing concentration of the enzyme catalase? Hypothesis The rate of oxygen production increases as the concentration of the micro-organism yeast, which is the source of the enzyme, increases. Variables Independent Variable: Concentration of yeast Dependent Variable: Rate of oxygen production Controlled Variables: Volume of hydrogen peroxide, mass of hydrogen peroxide, temperature and pressure Apparatus Instant dry yeast Measuring cylinder Boiling tube Delivery tube Ozanalp Eryılmaz 11/B Stopper Pipette 5 cm3 hydrochloric acid 100 cm3 beakers Stopwatch Warning Hydrogen peroxide may cause burns to skin and clothing. Wash off any spillages using plenty of water. Method 1. Stir the yeast and place in the boiling tube. 2. Put the stopper into the boiling tube but do not place the delivery tube under the measuring cylinder yet. 3. Fill the syringe with 1 ml 10% hydrogen peroxide and attach to the syringe needle. 4. Add the hydrogen peroxide to the yeast suspension. Place the delivery tube under the measuring cylinder and start a stopwatch. 5. Record the volume of oxygen collected every minute until no more is produced or until the measuring cylinder is full. 6. Repeat with different concentrations of yeast. (Maximum 2 gr of yeast) 7. Record your results in a table. 8. Calculate the rate of oxygen production for each concentration and plot your results as a graph. Ozanalp Eryılmaz 11/B DATA COLLECTION AND PROCESSING Mass of yeast / g 0.25 0.50 0.75 1.00 1.25 Initial volume / cm3 50 50 50 50 50 Final volume / cm3 62 69 77 87 93 Volume difference / cm3 12 19 27 37 43 50 45 40 35 30 Amount of Enzyme (g) 25 Volume Difference (cm3) 20 15 10 5 0 1 2 3 4 5 CONCLUSION AND EVALUATION Hydrogen peroxide breaks down into water and oxygen with the help of catalase. Yeast, containing the enzyme necessary for the reaction to take place, in the experiment catalyses this redox reaction. When the concentration of yeast was increased, the rate of oxygen production increased as well. Therefore, it can be concluded that the enzyme concentration is directly proportional to the rate of oxygen production. In the experiment, there could be inaccuracy caused by a number of experimental errors. First of all, the temperature of the boiling tube, in which yeast was placed, was not measured. A thermometer (with an uncertainty of 1°C) should be used to record whether the Ozanalp Eryılmaz 11/B temperature of the boiling tube is kept constant throughout the experiment. Besides, a pipette (with an uncertainty of 0.5 cm³) was used to measure the volume of hydrogen peroxide and a measuring cylinder (with an uncertainty of 1 cm³) to record the volume of oxygen collected. Finally, a stopwatch was used to record the rate of oxygen production and it has an uncertainty of about 0.2 s due to human reaction time at the start and stop moments. These random errors can be minimised by making use of the equipment with more precision. Repeating the experiment several times (at least five times) is a suitable solution to decrease human error as well.