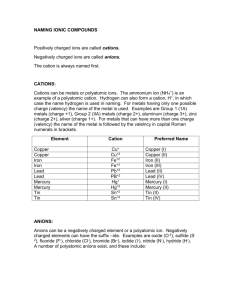
cations
advertisement

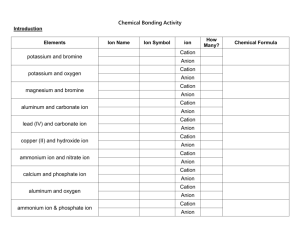
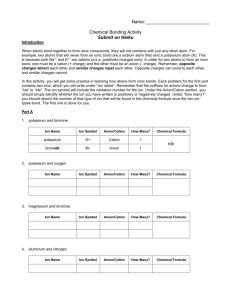
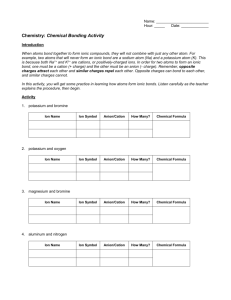
Chemistry: Chemical Bonding Activity Introduction In this activity, you will get some practice in learning how atoms form ionic bonds. Elements potassium and bromine potassium and oxygen magnesium and bromine aluminum and carbonate ion lead (IV) and carbonate ion copper (II) and hydroxide ion ammonium ion and nitrate ion calcium and phosphate ion aluminum and oxygen ammonium ion & phosphate ion Ion Name Ion Symbol ion Cation Anion Cation Anion Cation Anion Cation Anion Cation Anion Cation Anion Cation Anion Cation Anion Cation Anion Cation Anion How Many? Chemical Formula Use the pieces to make formula units for five more compounds, record the formulas below and get your teacher’s initials. Formula Unit of Compound 1. 4. 2. 5. 3. Questions 1. What is the overall charge on all of your formula units? 2. If you need more than 1 of a cation or anion to balance the formula, how is the number shown in the formula? 3. How is the formula written differently for compounds containing polyatomic ions? 4. Do cation pieces fit with other cation pieces? Explain why in terms of ions and charge, not the way they are cut. 5. Do anion pieces fit with other anion pieces? Explain why in terms of ions and charge, not the way they are cut. 6. Do metals form anions or cations? 7. Do nonmetals form anions or cations? 8. Write the chemical formula that results when the following pairs of ions combine to form an ionic bond. a. Sr2+ and O2d. Li1+ and Cl1b. Mn4+ and O2- e. Cs1+ and S2- c. K+ and SO42- f. Al3+ and SO42-