bond
advertisement

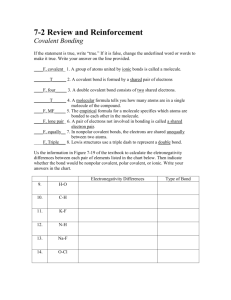
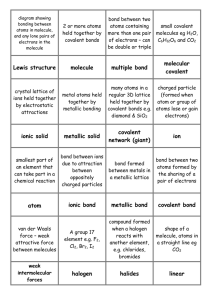
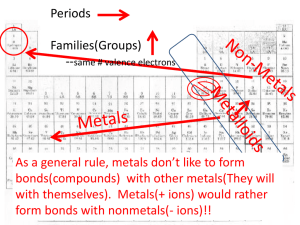
Chapter 5 Section 3 I. How do Covalent bonds form a. The chemical ______ formed when 2 atoms _______ electrons, usually between 2 ____________ b. Most nonmetals ______bond with another _______ of the ________ element c. The ______ that holds atoms together in a _________ bond is the attraction of each atom’s _________ for the shared _______ of electrons d. A MOLECULE IS A ___________ GROUP OF ATOMS __________ BY COVALENT BONDS. e. Except for ___________, the number of covalent bonds that nonmetals form _________ the number of electrons needed to make a ________ of 8, hydrogen only needs ____, Figure 15, page 167 f. Double and triple bonds can form when _______ share _________ one pair of electrons, double 2 pairs, triple 3 pairs. i. Figure 16, page 168 II. Molecular Compounds a. IS a __________ that is composed of ___________ that are covalently bonded b. Have ________ melting points and boiling points and they _______ conduct electricity when __________ in water. Math skills, page 169 c. Less heat is needed to _______ a covalent bond, that’s why most molecular compounds are ______________ at room temperature d. Do not conduct electricity because no charged __________ are available to move. Even when they are __________ they are ______conductors e. __________ Sharing of electrons f. Atoms of some elements pull _____________ on shared electrons than do atoms of other elements. As a result, the electrons are pulled more __________ atom, causing the bonded atoms to have slight electrical charge. g. These charges are _________ strong as ions III. Polar bonds: A __________bond in which electrons are shared ____________ IV. Non-polar bond: covalent bond in which the electrons are shared ____________. a. See figure 17, page 170 V. Polar bonds in molecules a. A molecule with ___________ bonds will itself be non-polar. But, a molecule may contain polar bonds and still be non-polar overall. b. A carbon dioxide molecule is a non-polar molecule because it’s a ___________ shape. In contrast a water molecule is polar because of its _____________. The bent shape allows the O end to have a slight negative charge and the H₂ end to have a slight positive charge c. Attraction between molecules i. Polar molecules require ____________ to break the bond than non-polar molecule


![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)