Study_guide_on_matter_and_its_properties
advertisement

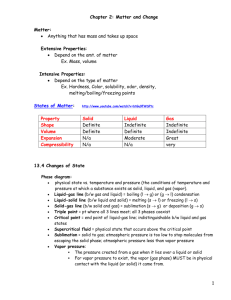
Matter Properties and change Study Guide Name: ________________ Date: ______________ 1. The water cycle Science textbook: pages B48-B49. Water cycle: The continuous movement of water between Earth’s surface and the air, changing from liquid to gas to liquid. Steps of the water cycle: 1. Evaporation: The process in which a liquid changes into a gas (water vapor) 2. Transpiration: The process by which plants release water vapor into the atmosphere through their leaves. 3. Condensation: The process in which a gas (water vapor) changes into a liquid. 4. Precipitation: Any form of water particles (rain, snow, hail or sleet) that fall from the sky. 5. Groundwater: On land some of the precipitation seeps into the ground and is stored as groundwater. 6. Runoff: Water that flows downhill. The main source of water in the water cycle is the oceans. 2. Matter (Chapter 12 in the textbook, lesson 2 and 3). Matter: Anything that has a mass and occupies space. Matter has 3 states: solid, liquid and gas. Mass: The amount of matter in an object. Mass is often measured in kg (kilograms or grams). Volume: How much space a sample of matter takes up. Volume is often measured in mL (milliliters: mL). Weight: The force of gravity between the Earth and an object. The weight is measured in N (Newtons). On the moon you would have the same mass, but you would weigh less than on Earth because the mass of the moon is less than Earth, so the force of gravity between your body and the moon would be less. Density: The quantity of matter in an object. How massive an object is for its size. It compares an object mass with its volume. If an object is denser than water, it will sink. If it is lighter than the water density, it will float. Matter is made up of atoms. Atom: Tiny particles that cannot be cut into smaller pieces. Atoms contain 3 kinds of particles in their center, called the nucleus: Protons (positive electric charge), neutron (no electric charge) and electrons (negative electric charge) that move around the nucleus. Atoms join together to form molecules. Molecule of elements contain only one kid of element. Examples: O2 (Oxygen), N2 (Nitrogen). Element: One kind of atom. Pure substance. Elements cannot be further broken down into other substances. There are 112 elements. Examples: Copper, Aluminum, Mercury. Compounds: 2 or more elements that combine form a compound. Compounds are made up of molecules that have different kinds of atoms joined together. Compounds are different from the elements that make them. Example: H2O (water), CO2 (carbon dioxide) 3. Matter and its properties Matter has 2 types of properties: Quantitative and qualitative. Qualitative properties Color Texture Appearance Smell Taste Comparison of some properties of solids, liquids and gas: Types of matter Solids Shape Definite shape. Shape does not change Liquids Indefinite shape. Liquids change shape. Indefinite shape. Gas Quantitative properties I can measure them. Mass Volume Temperature Density Volume Definite volume. The volume in solids does not change. It stays the same. Definite volume. The volume does not change. It stays the same. Indefinite volume. They fill the whole recipient. Mass Definite mass. The mass does not change. Definite mass. The mass does not change. Definite mass. The mass does not change. Another property of matter is temperature. I can measure temperature in Celsius or Fahrenheit. Celsius Fahrenheit Water boils At 100 C At 212 F Water freezes At o C At 32 F 4. Changes of matter Matter can change. Matter at the beginning A liquid (water) A liquid (water) A solid (ice) A gas (water vapor) Matter transforms into another type of matter A gas (water vapor) A solid (ice) A liquid (water) A liquid (water) Name Evaporation/vaporization freezing Melting Condensation