docx - Swissethics
advertisement

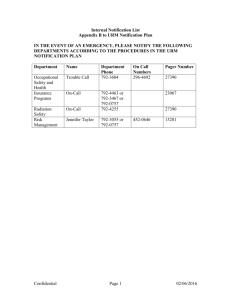
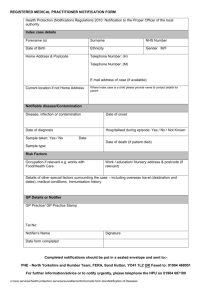
swissethics Schweizerische Ethikkommissionen für die Forschung am Menschen Commissions d’éthique suisses relative à la recherche sur l'être humain Commissioni etiche svizzere per la ricerca sull'essere umano Swiss Ethics Committees on research involving humans Notification and reporting to the ethics committee from 1 January 2014 (Author: Zurich CEC [Clinical Ethics Committee]) for clinical trials [Clinical Trials Ordinance; KlinV-ClinO] Reporting of safety and protective measures see Article 37 KlinV-ClinO: Notification to the EC within 7 days Trials of medical devices: within 2 days Completion, discontinuation or interruption of the clinical trial See Article 38 KlinV-ClinO: Notification of completion to the EC within 90 days Notification of discontinuation or interruption to the EC within 15 days Final report to the EC: within one year of completion or discontinuation Serious adverse events (SAEs) in clinical trials of medicinal products See Article 40 KlinV-ClinO: Unless otherwise specified in the protocol, SAEs with fatal consequences within 7 days (to the local EC for local events, to the responsible EC for all events in Switzerland). Suspected unexpected serious adverse reactions, SUSARs) See Article 41 KlinV-ClinO: SUSARs with fatal consequences within 7 days, other SUSARs within 15 days (to the local EC for local events, to the responsible EC for all events in Switzerland). Serious adverse events (SAEs) in clinical trials of medical devices See Article 42 KlinV-ClinO: In Category C trials, SAEs where a connection is suspected with the investigational device or intervention, within 7 days (to the local EC for local events, to the responsible EC for all events in Switzerland). Serious adverse events (SAEs) that may be related to the intervention under investigation in other clinical trials See Article 63 KlinV-ClinO: Notification of responsible EC within 15 days. Reporting on the safety of participants See Article 43 KlinV-ClinO: Annual list of global events (Annual Safety Report) The Annual Safety Report to the responsible EC must also report any changes that do not require approval (i.e. all changes that are not significant according to Art. 29 KlinV-ClinO). Notification and reporting version of 14.03.2014 page 1/2 swissethics Schweizerische Ethikkommissionen für die Forschung am Menschen Commissions d’éthique suisses relative à la recherche sur l'être humain Commissioni etiche svizzere per la ricerca sull'essere umano Swiss Ethics Committees on research involving humans Notification and reporting to the ethics committee from 1 January 2014 for research projects not involving clinical trials [Human Research Ordinance; HFV-HRO] Research with human subjects associated with measures for sampling biological material or the collection of health-related personal data Safety and protective measures see Article 20 HFV-HRO: Notification of the EC within 7 days Serious events See Article 21 HFV-HRO Notification within 7 days (to the local EC for local events, to the responsible EC for all events in Switzerland). Completion or discontinuation of a research project See Article 22 HFV-HRA Notification of the EC within 90 days Further use of biological material and health-related personal data for research purposes See Article 36 of HFV-HRO: Change of project leader Prior notification of the EC Completion or discontinuation of the research project Notification of the EC within 90 days Further use of biological material and health-related personal data for research purposes in the absence of informed consent according to Article 34 HFG [Human Research Act, HRA] See Article 40 HFV-HRO: Changes to the information given in the authorisation Prior notification of the EC Completion or discontinuation of the research project Notification of the EC within 90 days Research on deceased persons (Article 43 HFV-HRO) See Article 43 HFV-HRO: Change of project leader Prior notification of the EC Research projects involving deceased persons undergoing artificial respiration Significant changes to the research protocol Prior notification of the EC Abschluss oder Abbruch des Forschungsprojekts Notification of the EC within 90 days Notification and reporting version of 14.03.2014 page 2/2