SI - AIP FTP Server
advertisement
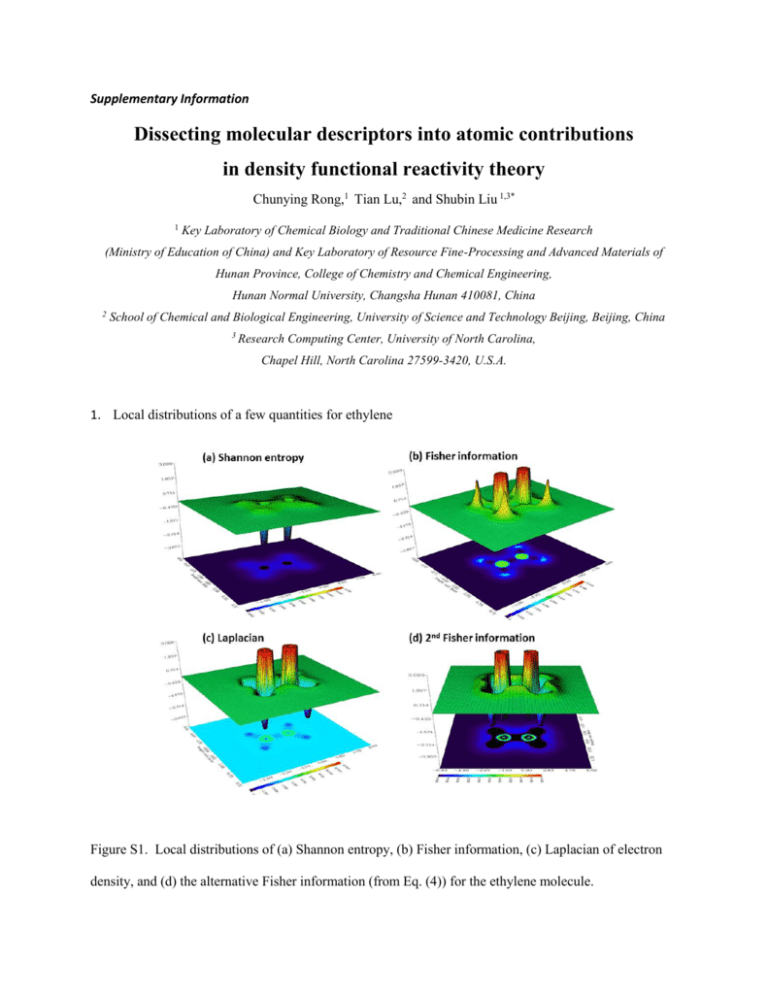
Supplementary Information Dissecting molecular descriptors into atomic contributions in density functional reactivity theory Chunying Rong,1 Tian Lu,2 and Shubin Liu 1,3* 1 Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research (Ministry of Education of China) and Key Laboratory of Resource Fine-Processing and Advanced Materials of Hunan Province, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha Hunan 410081, China 2 School of Chemical and Biological Engineering, University of Science and Technology Beijing, Beijing, China 3 Research Computing Center, University of North Carolina, Chapel Hill, North Carolina 27599-3420, U.S.A. 1. Local distributions of a few quantities for ethylene Figure S1. Local distributions of (a) Shannon entropy, (b) Fisher information, (c) Laplacian of electron density, and (d) the alternative Fisher information (from Eq. (4)) for the ethylene molecule. 2. Figures S2 and S3 (see SI) are results for the H-O-H bending of the water molecule, where we displayed profiles of the molecular and atomic values for Fisher information and Shannon entropy (Fig. S2), plus the strong linear correlations for these quantities (Fig. S3). As the angle is bended from 50 to 180, molecular and atomic values of Fisher information keep decreasing (Fig. S2a), with the hydrogen values fall off in a rather slow pace. For the Shannon entropy, the same pattern is seen for the molecular value and for the hydrogen atomic value, but for oxygen, its atomic Shannon entropy keeps increasing as the bond angles increases from 50 to 180. Again, we can understand this result from the viewpoint of what Shannon entropy measures, i.e., the spatial delocalization. As the angle enlarges, there is more space for oxygen atom to delocalize its electron distribution, leading to the increase of the entropy. In Fig. S3, strong linear correlations from atomic values of these two quantities with the correlation coefficient larger than 0.90 are displayed. They are atomic values between H and O atoms of Shannon entropy (Fig. S3a) and Fisher information (Fig. S3d), atomic values of Shannon entropy and Fisher information between hydrogen and oxygen atoms (Figs. S3b and S3c). No strong linear correlation was found at the molecular level nevertheless. Figure S2. Molecular and atomic profiles of (a) Fisher information and (b) Shannon entropy for the water molecule (black), oxygen atom (red) and hydrogen atom (green) in water molecule as a function of the H-O-H angle changed from 50 to 180. 3. Strong linear correlations among atomic contributions of Shannon entropy and Fisher information obtained from water molecule Figure S3. Strong linear correlations among atomic contributions of Shannon entropy and Fisher information obtained from water molecule when the H-O-H angle is rotated from 50 to 180. 4. Strong linear correlations among molecular and atomic values of Shannon entropy and Fisher information obtained from the ethane molecule Figure S4. Strong linear correlations among molecular and atomic values of Shannon entropy and Fisher information obtained from the ethane molecule as the H-C-C-H dihedral angle is rotated from 0 to 360.








