Density and the Particle Theory of Matter
advertisement
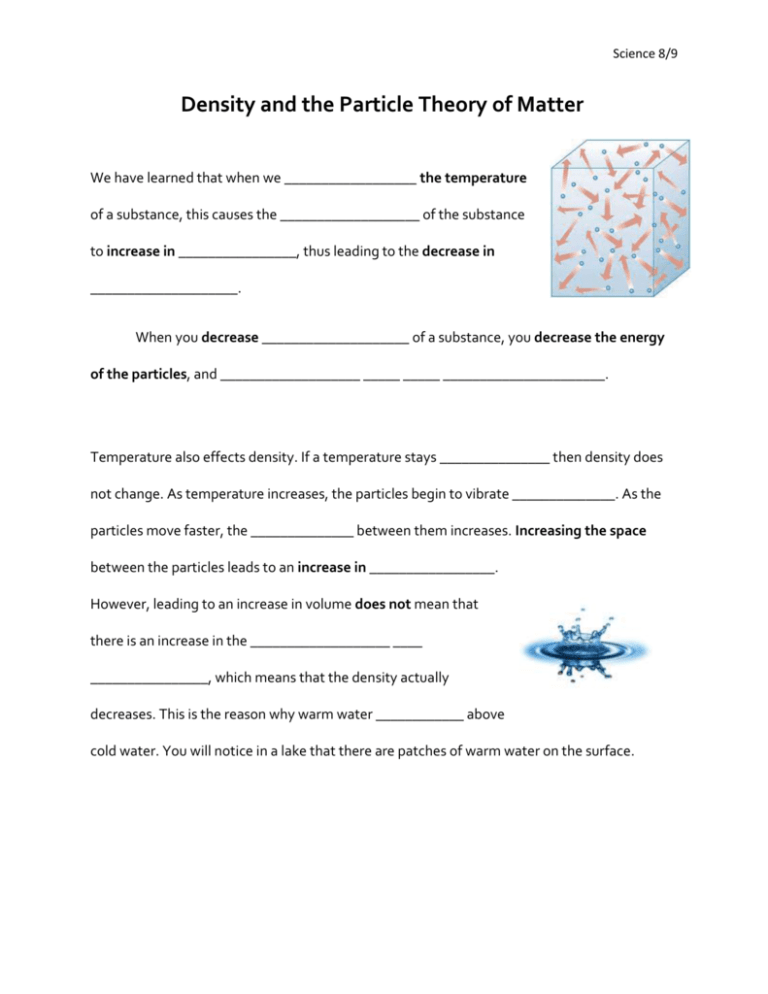
Science 8/9 Density and the Particle Theory of Matter We have learned that when we __________________ the temperature of a substance, this causes the ___________________ of the substance to increase in ________________, thus leading to the decrease in ____________________. When you decrease ____________________ of a substance, you decrease the energy of the particles, and ___________________ _____ _____ ______________________. Temperature also effects density. If a temperature stays _______________ then density does not change. As temperature increases, the particles begin to vibrate ______________. As the particles move faster, the ______________ between them increases. Increasing the space between the particles leads to an increase in _________________. However, leading to an increase in volume does not mean that there is an increase in the ___________________ ____ ________________, which means that the density actually decreases. This is the reason why warm water ____________ above cold water. You will notice in a lake that there are patches of warm water on the surface. Science 8/9 Density Review Questions 1. Use the particle theory to describe what is happening to the density of a substance when it is cooled. 2. Most substances increase their density as they move from a gas to liquid to a solid. Water is an exception, as it actually becomes less dense as it freezes. Without measuring how would you know that ice is less dense than water? 3. How do you think temperature affects the density of a fluid such as air in a balloon? 4. Calculate the density of 27.1 g of mercury that displaces 2.0 mL of water. 5. A fluid is not only a liquid but also a gas, therefore, what does it mean to be a fluid? 6. Indicate two ways you can determine viscosity of a fluid.