Landmark asthma studies
advertisement
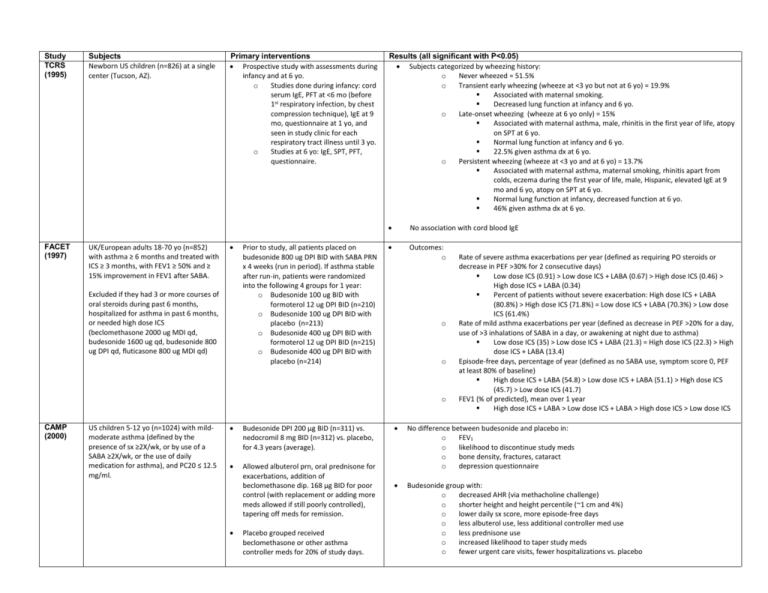
Study TCRS (1995) FACET (1997) Subjects Newborn US children (n=826) at a single center (Tucson, AZ). UK/European adults 18-70 yo (n=852) with asthma ≥ 6 months and treated with ICS ≥ 3 months, with FEV1 ≥ 50% and ≥ 15% improvement in FEV1 after SABA. Primary interventions Prospective study with assessments during infancy and at 6 yo. o Studies done during infancy: cord serum IgE, PFT at <6 mo (before 1st respiratory infection, by chest compression technique), IgE at 9 mo, questionnaire at 1 yo, and seen in study clinic for each respiratory tract illness until 3 yo. o Studies at 6 yo: IgE, SPT, PFT, questionnaire. Excluded if they had 3 or more courses of oral steroids during past 6 months, hospitalized for asthma in past 6 months, or needed high dose ICS (beclomethasone 2000 ug MDI qd, budesonide 1600 ug qd, budesonide 800 ug DPI qd, fluticasone 800 ug MDI qd) Prior to study, all patients placed on budesonide 800 ug DPI BID with SABA PRN x 4 weeks (run in period). If asthma stable after run-in, patients were randomized into the following 4 groups for 1 year: o Budesonide 100 ug BID with formoterol 12 ug DPI BID (n=210) o Budesonide 100 ug DPI BID with placebo (n=213) o Budesonide 400 ug DPI BID with formoterol 12 ug DPI BID (n=215) o Budesonide 400 ug DPI BID with placebo (n=214) Results (all significant with P<0.05) Subjects categorized by wheezing history: o Never wheezed = 51.5% o Transient early wheezing (wheeze at <3 yo but not at 6 yo) = 19.9% Associated with maternal smoking. Decreased lung function at infancy and 6 yo. o Late-onset wheezing (wheeze at 6 yo only) = 15% Associated with maternal asthma, male, rhinitis in the first year of life, atopy on SPT at 6 yo. Normal lung function at infancy and 6 yo. 22.5% given asthma dx at 6 yo. o Persistent wheezing (wheeze at <3 yo and at 6 yo) = 13.7% Associated with maternal asthma, maternal smoking, rhinitis apart from colds, eczema during the first year of life, male, Hispanic, elevated IgE at 9 mo and 6 yo, atopy on SPT at 6 yo. Normal lung function at infancy, decreased function at 6 yo. 46% given asthma dx at 6 yo. No association with cord blood IgE Outcomes: o o o o CAMP (2000) US children 5-12 yo (n=1024) with mildmoderate asthma (defined by the presence of sx ≥2X/wk, or by use of a SABA ≥2X/wk, or the use of daily medication for asthma), and PC20 ≤ 12.5 mg/ml. Budesonide DPI 200 μg BID (n=311) vs. nedocromil 8 mg BID (n=312) vs. placebo, for 4.3 years (average). Allowed albuterol prn, oral prednisone for exacerbations, addition of beclomethasone dip. 168 μg BID for poor control (with replacement or adding more meds allowed if still poorly controlled), tapering off meds for remission. Placebo grouped received beclomethasone or other asthma controller meds for 20% of study days. Rate of severe asthma exacerbations per year (defined as requiring PO steroids or decrease in PEF >30% for 2 consecutive days) Low dose ICS (0.91) > Low dose ICS + LABA (0.67) > High dose ICS (0.46) > High dose ICS + LABA (0.34) Percent of patients without severe exacerbation: High dose ICS + LABA (80.8%) > High dose ICS (71.8%) = Low dose ICS + LABA (70.3%) > Low dose ICS (61.4%) Rate of mild asthma exacerbations per year (defined as decrease in PEF >20% for a day, use of >3 inhalations of SABA in a day, or awakening at night due to asthma) Low dose ICS (35) > Low dose ICS + LABA (21.3) = High dose ICS (22.3) > High dose ICS + LABA (13.4) Episode-free days, percentage of year (defined as no SABA use, symptom score 0, PEF at least 80% of baseline) High dose ICS + LABA (54.8) > Low dose ICS + LABA (51.1) > High dose ICS (45.7) > Low dose ICS (41.7) FEV1 (% of predicted), mean over 1 year High dose ICS + LABA > Low dose ICS + LABA > High dose ICS > Low dose ICS No difference between budesonide and placebo in: o FEV1 o likelihood to discontinue study meds o bone density, fractures, cataract o depression questionnaire Budesonide group with: o decreased AHR (via methacholine challenge) o shorter height and height percentile (~1 cm and 4%) o lower daily sx score, more episode-free days o less albuterol use, less additional controller med use o less prednisone use o increased likelihood to taper study meds o fewer urgent care visits, fewer hospitalizations vs. placebo SMART (2006) US subjects ≥12 yo with asthma (as judged by study physician) and currently receiving prescription asthma medication. Excluded if ever used LABA, pregnancy, significant systemic disease, or current use of betablocker. Multicenter (over 6000 sites). Recruitment in 2 phases (~3 years each): phase 1 via media advertising, phase 2 directly by study investigators. PACT (2007) Salmeterol 42 μg MDI BID added to current medications (n=13,176) vs. placebo (n=13,179) added to current medications, for ~6.5 months (28 weeks). GlaxoSmithKline terminated the study early due to preliminary findings in AAs and difficulties in enrollment (but predefined criteria for study termination were not met at the interim analysis). Primary endpoint: occurrence of respiratory-related (includes asthma-related) deaths or respiratoryrelated life-threatening experiences, defined as intubation and mechanical ventilation. o No difference between salmeterol and placebo for total population. o AA/salmeterol subgroup with statistically significant relative risk (4) for primary endpoint vs. AA/placebo o No difference between phase 1 and phase 2 for total population. o AA/salmeterol/no ICS subgroup with statistically significant RR (5.6) for primary endpoint vs. AA/placebo/ICS Secondary endpoints: o Total pop/salmeterol with statistically significant RR for the following: combined asthma-related death or life-threatening experience (1.7), respiratory-related death (2.1), asthma-related death (4.3) o Caucasian subgroup with no difference between salmeterol and placebo. o AA/salmeterol subgroup with statistically significant RR for the following: combined asthma-related death or life threatening experience (4.9), combined all-cause death or life-threatening experience (2.1). o By phase of trial: Total pop/salmeterol with statistically significant RR (1.8) for combined asthma-related deaths or life-threatening experiences during phase 1; No difference in secondary endpoints during phase 2. o By baseline ICS use: Total pop/salmeterol/no ICS with statistically significant RR (2.39) for combined asthma-related death or life-threatening experience. Caucasian subgroup with no difference between ICS and no ICS. AA/salmeterol/no ICS subgroup with statistically significant RR (10.4) for combined asthma-related death or life-threatening experience. Primary outcome: percent of asthma control days during study (defined as a day w/o albuterol, oral steroids, additional controller medication, day/night sx, unscheduled PCP/ER visits, hospitalizations, school absence due to asthma) o Fluticasone (64.2 %) = combination (59.6 %) > montelukast (52.5 %) Secondary outcomes (change vs. baseline): o Change in FEV1: Fluticasone (+6.3 %) = combination (+3.6 %) > montelukast (-0.58 %) o Decrease in eNO: Fluticasone (-59.1 %) > combination (-22.9 %) > montelukast (-18.9 %) o AHR via methacholine challenge: Fluticasone > combination = montelukast o Proportion of subjects not requiring oral steroids during study: Fluticasone (~60%) = combination (~48%) > montelukast (~42%) o Growth: no difference between treatments Contacted by phone Q4 weeks to assess study endpoints. Baseline ICS use by 47% overall (49% in Caucasians and 38% in African Americans). Data for asthma history and current nocturnal symptoms indicate greater disease severity at baseline in the AA subgroup vs. Caucasian. US children 6-13 yo (n=285) with mildmoderate persistent asthma (via reported sx, SABA use, or peak flow <80% calculated), FEV1 ≥ 80%, PC20 ≤ 12.5 mg/ml. Excluded if history of life threatening asthma, >4 courses oral steroids or 2 hospitalizations in past year. Fluticasone 100 μg DPI BID (n=86) vs. “combination” fluticasone/salmeterol 100/50 μg DPI QAM + salmeterol 50 μg DPI QHS (n=81) vs. montelukast 5 mg PO QHS (n=83), for 48 weeks (11 months). LOCSS (2007) US patients ≥6 yo (n=500) with asthma was acceptably controlled after 4 to 6 weeks of open-label treatment with fluticasone propionate (Flovent Diskus, GlaxoSmithKline) Inclusion criteria for enrollment in the run-in period: age ≥6 yo, and FEV1≥60% predicted before SABA, and ≥12% improvement in FEV1 post-SABA, or methacholine PC20 ≤8 mg. 6 week run-in period with fluticasone 100 μg DPI BID (run-in period). If asthma stable, randomized to following groups: continue fluticasone 100 μg DPI BID (n=169), fluticasone 100 μg + salmeterol 50 μg DPI QHS (n=165), or montelukast 510 mg PO QHS (n=166) for 16 weeks. Primary outcome: o Time to treatment failure, defined as: ER/hospital vist, need for systemic corticosteroids or open-label ICS use, fall in pre-SABA FEV1 to more than 20% below baseline value; decrease in AM PEFR >35% below baseline value on 2 consecutive days; use of ≥10 puffs/day of SABA for 2 consecutive days (excluding exercise premed); refusal of the patient to continue because of lack of satisfaction with treatment; or judgment by a physician that the patient should stop treatment for reasons of safety. fluticasone (20.2%) = fluticasone + salmeterol (20.4%) > montelukast (30.3%) Secondary outcomes: o FEV1 %predicted (pre-SABA): fluticasone + salmeterol (91.8%) = fluticasone (91.1%) > montelukast (88.8%) o Morning PEFR %predicted: fluticasone + salmeterol (99%) > fluticasone (96.2%) = montelukast (94.7%) o Asthma symptom free days: fluticasone (85.8%) = fluticasone + salmeterol (82.7%) = montelukast (78.7%) o Days with use of SABA fluticasone (18.2%) = fluticasone + salmeterol (17.1%) = montelukast (22.9%) Primary outcome: o Response to each of the 3 step-up therapies was judged on the basis of: Total amount oral steroids required Asthma control days (during latter 12 weeks of each 16 week period). ACD defined as day with w/o albuterol, day/night sx, unscheduled visit to health care provider, or peak flow <80% of best) Change in FEV1 A treatment considered to be better if needed at least 180 mg less oral steroids; if not, better if more asthma control days; if not, better if FEV1 improved at least 5%. If no difference in any parameter, treatments considered to be same. o 98% of patients had a differential response, with most responding to LABA step-up best. Probability of best response: add LABA > increase ICS = add LTRA. o Although LABA step-up most likely to provide the best response overall, some children had a best response to increased ICS or LTRA step-up. Analysis of predictors of differential response: o Response affected by: Asthma control test >19, more likely to have best response to LABA. Race - Hispanic and whites most likely to have a best response to LABA, least to ICS. AA patients equally likely to have a best response to LABA or ICS and least to LTRA. Eczema status – without eczema more likely to have best response to LABA. o Response not affected by: AHR (PC20 >1.5 vs <1.5 mg/mL), FeNO (>10 vs <10 ppb), 2 receptor genotype (Arg/Arg vs Arg/Gly vs Gly/Gly), age, sex, sensitization to perennial allergens, baseline FEV1 (>90% vs <90%), bronchodilator reversibility (>10% vs <10% FEV1), previous use or nonuse of a controller med, baseline number of asthma control days, and number of recent asthma exacerbations (0 vs ≥1). Inclusion criteria after the run-in period: adequate adherence, a pre-SABA FEV1 ≥ 80% predicted, Asthma Control Questionnaire score of <1.5 (0-6 scale, lower is better); <16 puffs of SABA per week during final 2 weeks of the run-in period (excluding exercise premedication); no hospitalization, urgent medical care (for asthma), oral corticosteroid use, or use of additional asthma medication during the run-in period; and an absence of febrile illness within the previous 24 hours. BADGER (2010) US children/teens 6-17 yo (n=165) with mild-moderate asthma (as defined by NAEPP), FEV1 ≥ 60%, and FEV1 increase ≥ 12% after SABA or PC20 ≤ 12.5 mg/ml. 28 weeks prior to the treatment period, their asthma was poorly controlled on fluticasone 100 μg DPI BID. 48 week / 3 period (16 weeks each) crossover trial. During each 16 week period, subjects received the following step-up therapies: increase fluticasone to 250 μg DPI BID, or switch to fluticasone/salmeterol 100/50 μg DPI BID, or add montelukast 5-10 mg PO QD (to fluticasone 100 μg DPI BID). Allowed albuterol prn, oral prednisone for exacerbations. TALC (2010) US adults ≥18 yo with asthma confirmed by bronchodilator reversibility or BHR, FEV1 ≥ 40% of predicted value, and nonsmoking status (<10 pack-years). Enrolled in TALC study if after 4 week run-in period with beclomethasone 80 μg HFA MDI BID (all other asthma medications stopped) patients with FEV1 <70% predicted or if during final 2 weeks of run-in period they had sx ≥6 days/wk or used SABA ≥6 days/wk or were awakened by asthma ≥2 nights/wk. Prospective 3-way, double-blind, triple crossover trial (n=210) Patients were treated for a 14-wk period with: o Beclomethasone 80 μg HFA MDI BID + tiotropium bromide DPI 18 μg QAM, or o Beclomethasone 160 μg HFA MDI BID, or o Beclomethasone 80 μg HFA MDI BID + salmeterol 50 μg DPI BID Between each 14 wk period, there was a 2 wk washout period with beclomethasone 80 μg HFA MDI BID. Primary end point: morning PEF o tiotropium = salmeterol > double ICS Secondary end points: o Evening PEF tiotropium > salmeterol > double ICS o FEV1 (pre-bronchodilator) tiotropium > salmeterol = double ICS o Asthma-control days (day without symptoms and without use of SABA) tiotropium = salmeterol > double ICS o Asthma exacerbations with PO or IV steroids double ICS (13) > tiotropium (7) > salmeterol (5) o Unscheduled visits for asthma symptoms double ICS (6) > tiotropium (2) = salmeterol (2) PEDIATRIC ASTHMA PREVENTION STUDIES Study ETAC (2002) Subjects Primary interventions Conclusions (all significant with P<0.5) UK children 1-2 yo with active AD for at least 1 month. Placebo (n=275) vs. cetirizine 0.25 mg/kg PO BID (n=274) for 18 month treatment period, then observation for 18 months. During the study, choice of treatment for any conditions manifested by the children was left to the clinical investigators/other physicians managing the children. Primary end point: time to the onset of asthma (defined as 3 episodes separated by at least 7 days, either of nocturnal cough with sleep disturbance lasting for at least 3 consecutive nights, wheezing, or a combination of the 2) o No significant difference between placebo and cetirizine groups in probability of developing asthma after 36 mo. Median time to asthma onset was 27.1 mo for placebo and 26.5 months for cetirizine. Analysis of subgroups with elevated grass pollen sIgE (>0.35): elevated GP/placebo >> others Analysis of subgroups with elevated dust mite sIgE (>0.35): elevated DM/placebo > elevated DM/cetirizine > negative DM/cetirizine > negative DM/placebo Increased relative risk for developing asthma associated with elevated IgE to egg (RR 1.4), grass pollen (1.7), DM (1.6), cat dander (1.5), but not milk (1.1) No difference in eczema improvement between groups. Overall trend toward improvement in eczema during the course of study in all treatment groups with all patterns of sensitivity. No significant difference between groups in adverse effects, including EKG (RR, QT). Development of asthma defined as recurrence of ≥2 out of 3 sx (cough, wheeze, SOB) within the previous 12 mo, with sx that are not triggered only by infections and clinically respond to SABA; not dependent on the amount of reversibility with SABA. After 3 years: o Excluded if wheezing episodes after 6 mo old, persistent nocturnal coughing, chronic pulmonary disease or other severe disorder likely to interfere with the study drug or observation of outcome, regular antihistamine use, oral cromoglycate use, or systemic steroids in the previous few weeks before recruitment. PAT (2002) European children 6-14 yo with AR to birch and/or grass (with positive SPT ≥3 mm and conjunctival challenge). Excluded if positive SPT to any allergen other than grass and/or birch, previous SCIT treatment, or asthma with need of daily medication. Study performed at 6 centers. Subcutaneous IT to birch and/or grass (maintenance doses: 12 μg Bet v 1, 20 μg Phl p 5, started before allergy season) (n=79) vs. no immunotherapy (n=72), for 3 years, then observed for 2 years. Both groups were allowed to take symptomatic medication, limited to loratadine, nasal levocabastine (antihistamine), ocular sodium cromoglycate, and nasal budesonide. In case of asthmatic symptoms, SABA and ICS could be given. Methacholine challenge done before allergy season (baseline), during season, and each winter for 3 years, and again at o o 24% developed asthma in IT group, 44% in controls. 5/6 centers concordant with similar results. AHR incrementally decreased each year in IT group, no change in controls. IT group with improved symptom scores for conjunctivitis after 1st year and rhinitis after 2nd year vs. control. After 5 years (3 years IT + 2 years observation): o 20% with asthma in IT group, 76% in controls (4 subjects lost to follow-up in each group). o No significant difference in AHR between groups. AHR decreased in both groups vs. baseline. o IT group with stable improved symptom scores for conjunctivitis and rhinitis vs. control, (similar to scores after 1st 3 years). 5 years. PEAK (2006) US children 2.4-3.6 yo (n=285) with positive asthma predictive index (frequent wheezing with one of the following: AD, AR, parent with asthma; OR 2 of the following: food allergy, wheezing unrelated to colds, eosinophilia). Fluticasone 88 μg BID via aerochamber/mask (n=143) vs. placebo (n=142) for 2 years, then observation off study medication for 1 year. Allowed albuterol prn, oral prednisolone for exacerbations, additional controller meds for poor control (e.g. more fluiticasone, montelukast). Additional controller meds used in 13% (fluticasone) and 21% (montelukast) of placebo group during 2 year treatment period. At end of 2 year treatment period: o Fluticasone group with more symptom free days (93% of treatment period v. 88%) less prednisolone use for exacerbations less singulair or additional fluticasone added for poor control longer time to 2nd course of prednisolone or addition of controller med decreased airway resistance measured via impulse oscillometry shorter height (-1.1 cm), with slower growth velocity during 1st year o No difference between groups in albuterol use time to 1st prednisolone course unscheduled physician visits and hospitalizations growth velocity during 2nd year At end of 3rd year (2 year treatment + 1 year observation period): o Fluticasone group with less additional fluticasone added for poor control (only during 1st 3 months of observation period) shorter height (-0.7 cm) at end of 3rd year, with increased “catch-up” growth velocity during 3rd year o No difference between groups in symptom-free days albuterol, montelukast, prednisolone use unscheduled physician visits and hospitalizations airway resistance via impulse oscillometry o Children with a positive response to fluticasone treatment were more likely (OR 2.32) to have increase in symptoms during observation period.










