gcb12153-sup-0012-SupportingInformation
advertisement

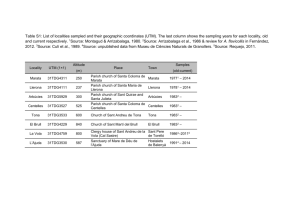
Supporting Information Stratigraphy, fauna and dating of the investigated caves The location of the investigated sites can be found in Fig. S1. Sesselfelsgrotte (Germany; 48° 55’ 60 N, 11° 48’ 00 E; see Figure S1) The Palaeolithic site Sesselfelsgrotte in southern Germany was excavated during 19641977 and 1988 and the analyses indicate that hominin occupation started at least during the early Weichselian (Freund, 1998, Richter et al., 2000). The Sesselfelsgrotte sequence includes a number of layers, some of which are yield mammal fossils. Layer K, which contained among other findings fossil Soricidae remains investigated in this study referred to as Sorex cf. araneus and Sorex cf. coronatus (Thomassen, 1996) see Table S1), was assigned to the end of MIS4 (~60 kyrs BP), the first maximum cooling of the Weichselian glacial (Böhner, 2009). Recent investigations of the small mammal fauna from the Sesselfelsgrotte show a visible decrease in abundance of indicators for cold climate and an increase of species which indicate more temperate climate (van Kolfschoten, in press). Interestingly, the Dshungarian hamster (Phodopus sungorus), a typical steppe dweller, that currently lives in Kazakhstan and southwest Siberia in a completely open landscape, is recorded with high abundance exclusively in layer K of the Sesselfelsgrotte sequence (van Kolfschoten, in press). This fits well with (Böhner, 2009), who placed layer K at the end of the first maximum cooling of the Weichselian glacial at ~ 60 kyrs BP (end of MIS 4) with extreme cold climatic conditions and subsequent warming at the beginning of MIS 3. Table S1 shows the current knowledge of the distribution of mammal remains in the Sesselfels sequence since MIS4. Nixloch cave (Austria; 47° 55’ 15 N, 14° 22’ 58 E; see Figure S1) Nixloch cave was excavated in 1986 and 1987 and contains six layers (A to F; Table S2). The small mammals studied were excavated from layer A, which is radiocarbon dated to 10.550±150 14C BP (VRI-1188), congruent with the Greenland Stadial 1 (Rabeder, 1992). The presence of small mammal species such as Ochotona pusilla, Dicrostonyx torquatus and Microtus gregalis, typical bioindicators for cold climate, support the placement of layer A into the Younger Dryas period. It should be noted that layer A might have been disturbed at the bottom, because of digging activities of humans and other mammals (Rabeder, 1992). Layer B is dated to 18.310±580 14 C BP (VRI-1030), representing the time of the Last Glacial Maximum (Rabeder, 1992). The age and genesis of the layers C to F are unknown. Gamssulzen cave (Austria; 47° 40’ 56 N, 14° 17’ 52 E; see Figure S1) Gamssulzen cave contains sediments of ~38.000-10.180 14 C BP (Rabeder, 1995) age ranging from the Weichselian Pleniglacial (a time with very rapid stadial/interstadial oscillations) to the Greenland Stadial 1 (Younger Dryas). This cave was excavated at three sites (Bärengruft, Bärengalerie and Entrance hall). The subfossil remains of small mammals are exclusively from the ”entrance hall”. The sediment bed containing the small mammals includes dates ranging from ~14.000-10.180 14 C BP (Rabeder, 1995) referring to ”Early” Late Glacial and Late Glacial ages. A mandible of Cricetus cricetus is dated to 10.792-11.087 14C BP (ETH-11569). Shifting sediments caused the occurrence of cave bear bones with 14 C ages from 38.0-27.5 kyrs 14 C BP close to the sediment bed with the small mammal remains. The sediment bed in the entrance hall, containing the microvertebrates , reveals the following mammal species: Talpa europaea, Sorex minutus, Sorex alpinus, Sorex cf. coronatus, Sorex macrognathus, Neomys anomalus, Marmota marmota, Cricetus cricetus, Microtus arvalis, Microtus agrestis, Microtus oeconomus, Microtus subterraneus, Microtus nivalis, Arvicola terrestris, Myodes glareolus, Apodemus sylvaticus, Lepus timidus, Vulpes vulpes, Martes martes, Mustela erminea, Ursus arctos, Alces alces, Capra ibex, and Rupicapra rupicapra (Frank et al., 1995, Nagel et al., 1995). The faunal composition of mollusks and small mammals of the ”entrance hall” refers to a Late Glacial age and suggests a warming event, which was very likely the GI1 (Bølling/Allerød warming) (Frank, 1995, Frank et al., 1995, Nagel et al., 1995, Rabeder, 1995). Results and Discussion Critical evaluation of species determination using mitochondrial DNA We would like to note that the presented results have to be treated with some caution as they are based solely on mitochondrial DNA. Therefore, our findings could also be explained by ancient introgression events or incomplete lineage sorting, especially as it has previously been shown that S. granarius shares its mitochondrial DNA with S. araneus, whereas its Y-Chromosome is closely related to S. coronatus (Yannic et al., 2008, Yannic et al., 2010). In a recent study (Yannic et al., 2010) showed that X-linked and other nuclear markers are congruent with the phylogenetic reconstruction using the Y-chromosome and thus concluded that the most plausible reason for the observed pattern is an ancient introgression of S. araneus mtDNA into S. granarius resulting in a complete replacement of the original S. granarius mtDNA. A similar scenario of mtDNA replacement is the polar bear (Edwards et al., 2011, Hailer et al., 2012). MtDNA introgressions among closely related species, resulting in the complete replacement of the original mtDNA in one species could be more common than generally assumed. However, misidentification of the fossil specimens is still the more parsimonious explanation, especially as the morphological similarities and ecological adaptations of the investigated taxa support our taxonomic assignments. More generally, though, definite species identifications cannot be provided without phylogenetic analysis of nuclear genes of the specimens investigated. However, retrieval of nuclear DNA strongly depends on the preservation of fossil remains and is still the exception rather than the rule in ancient DNA studies. Given the size and age of the studied specimens and our success rate of about 50% for obtaining mtDNA, we think that it is quite unlikely to retrieve sufficient ancient nuclear DNA for a more resolved phylogeny. Statistical analysis of the obtained measurements We observed a strong correlation between the height of the coronoid and the length of the processus condylaris in both S. araneus (cor=0.849091, 95% confidence interval: 0.7648882 -0.9047647; p-value <2.2e-16) and S. tundrensis (cor=0.9227777, 95% interval: 0.8712386 - 0.9541924; p-value <2.2e-16). Thus, we used only coronoid height for the subsequent statistical tests. The Levenes and the Brown-Forsythe Levene-type test were not significant for the studied measurements at a significance level of p < 0.01 (S. araneus: 0.03792 and S. tundrensis 0.0259). S. araneus The Shapiro-Wilk test showed no evidence against a normal distribution for the S. araneus samples (p-value: 0.1347). The one-way ANOVA shows that samples of S. araneus were not compatible with the null hypothesis that these samples are drawn from the same population (p-value: 6.68e- 7). The pairwise t-test with Bonferroni correction indicates significant differences in size between modern specimens and samples from NL and GS (p <0.01, Table S5). We found no significant differences between NL and GS, between NL and SF and between modern samples and SF (Table S5). S. tundrensis We rejected the null hypothesis of normally distributed measurements for S. tundrensis using the Shapiro-Wilk test (p-value: 2.58e-4). Similar to the one-way ANOVA for S. araneus, the Kruskal-Wallis test indicates a population difference in our S. tundrensis sampling (p-value 4.55e-7).The applied Mann-Whitney U test shows a significant size difference between modern samples and samples from NL and between samples from SF and from NL (tolerance level<0.01, Table S6). Some of the non significant p-values when comparing the sample from GS to other populations may have resulted from the small sample size (n=2) for GS as the individual measurements for GS fall within the size range of the samples from NL. We also found no significant differences between modern samples and samples from SF. In a second analysis we investigated the size difference between the specimens described in (Bobretsov et al., 2008) and our samples. This sampling includes forest inhabiting specimens that show a bigger body size and different coloration than tundra inhabiting specimens. Figure S8 shows a boxplot for the different populations investigated. The Brown-Forsythe Levene-type test was not significant for the combined data (p-value: 0.1518). The Kruskal-Wallis test showed a significant difference between the samples (pvalue:<2.2e-16). Like in the previous analysis, the Mann-Whitney U test showed nonsignificant p-values for comparisons of the GS samples set to other populations. In some cases this can be attributed to a small sampling size (Table S7). Tundra samples showed a significant difference to all other populations (with the exception of GS). We found no significant difference between samples from the mountains, plains and foothills. All these samples, however, differed significantly from NL, SF and our modern sampling (this study). Interestingly, samples from the tundra were significantly smaller in size than all other populations. Samples from the plain, the mountain and foothills were significantly bigger than our recent sampling and SF samples, but significantly smaller than samples from NL (and smaller than samples from GS; see Fig. S2). Thus, the observed size increase in samples from NL and GS even exceeds the size increase of the forest-form described in (Bobretsov et al., 2008). Supporting references Bannikova AA, Dokuchaev NE, Yudina EV, Bobretsov AV, Sheftel BI, Lebedev VS (2010) Holarctic phylogeography of the tundra shrew (Sorex tundrensis) based on mitochondrial genes. Biological Journal of the Linnean Society, 101, 721-746. Bobretsov A, Kupriyanova I, Petrov A, Demidova T, Shchipanov N (2008) A European forest for of Sorex tundrensis (Insectivora). Zoologicheskii zhurnal, 87, 841-849. Böhner U (2009) Die Schicht E3 der Sesselfelsgrotte und die Funde aus dem Abri I am Schulerloch, Steiner. Edwards CJ, Suchard MA, Lemey P et al. (2011) Ancient hybridization and an Irish origin for the modern polar bear matriline. Current Biology, 21, 1251-1258. Frank C (1995) Mollusca (Gastropoda) aus der Gamssulzenhöhle im toten Gebirge. Mitteilungen der Kommission für Quartärforschung der Österreichischen Akademie der Wissenschaften, 9, 53-59. Frank C, Kunst G, Mlikovsky J, Nagel D, Rabeder G, Rauscher K, Reiner G (1995) List of fossil fauna of Gamssulzenhöhle, Totes Gebirge, Upper Austria. Mitteilungen der Kommission für Quartärforschung der Österreichischen Akademie der Wisenschaften, 9, 51-52. Freund D (1998) Sesselfelsgrotte I: Grabungsverlauf und Stratigraphie., Saarbrücken, Saarbrücker Dr. und Verl. Hailer F, Kutschera VE, Hallström BM et al. (2012) Nuclear genomic sequences reveal that polar bears are an old and distinct bear lineage. Science, 336, 344-347. Nagel D, Rabeder G, Reiner G (1995) Insectivores and Rodents of the Late Glacial period of Gamssulzenhoehle in ”Totes Gebirge”, Upper Austria. Mitteilungen der Kommission für Quartärforschung der Österreichischen Akademie der Wisenschaften, 9. Rabeder G (1992) Standard profile and chronology of Nixloch cave sediments. Mitt. Komm. Quartaerforschung, 8, 223-225. Rabeder G (1995) Chronologie der Gamssulzenhöhle im Toten Gebirge (Oberösterreich). Mitteilungen der Kommission für Quartärforschung der Österreichischen Akademie der Wisenschaften, 9, 129-133. Richter D, Mauz B, Bohner U et al. (2000) Luminescence dating of the middle/upper Palaeolithic sites ‘Sesselfelsgrotte’and ‘Abri I Schulerloch’, Altmühltal, Bavaria. In: Neanderthals and modern humans—discussing the transition. Central Europe from 50.000--30.000 BP. (eds Orschiedt J, Weniger G-C) pp Page. Mettmann, Neanderthal Museum. Thomassen H (1996) De Midden Paleolithische kleine zoogdierfauna uit de Sesselfelsgrot (Zuid- Duitsland), met nadruk op de spitsmuizen (Mammalia, Insectivora, Soricidae). . Cranium, 13, 47-52. Van Kolfschoten T (in press) The smaller mammals from the Late Pleistocene sequence of the Sesselfelsgrotte., Stuttgart, Steiner Verlag. Yannic G, Basset P, Hausser J (2008) A new perspective on the evolutionary history of western European Sorex araneus group revealed by paternal and maternal molecular markers. Molecular Phylogenetics and Evolution, 47, 237-250. Yannic G, Dubey S, Hausser J, Basset P (2010) Additional data for nuclear DNA give new insights into the phylogenetic position of Sorex granarius within the Sorex araneus group. Molecular Phylogenetics and Evolution, 57, 1062-1071. Supporting Information Figure Legends Figure S1: Locations of the studied sites: (1) Sesselfels, (2) Nixloch and (3) Gamssulzen. Figure S2: Modern and ancient shrew jaws. (a) Buccal view of modern S. araneus, (b) lingual view of modern S. araneus, (c) buccal view of ancient S. araneus, (d) lingual view of ancient S. araneus, (e) buccal view of modern S. tundrensis, (f) lingual view of modern S. tundrensis, (g) buccal view of ancient S. tundrensis and (h) lingual view of ancient S. tundrensis. All modern samples are from the collection of the Museum of Natural History, Vienna and all ancient samples from the Department of Paleontology, University of Vienna. Photos by Rudolf Gold. Figure S3: Discriminant Analysis of Principal Component (DAPC) plot for the members of the Sorex araneus-group species. (1) S. cf. tundrensis, (2) S. macrognathus, (3) S. tundrensis, (4) S. araneus, (5) S. coronatus, (6) S. granarius, (7) S. satunini, (8) S. asper and (9) S. arcticus. Figure S4: Boxplots of the measurements obtained in this study and measurements from (Bobretsov et al., 2008) of the coronoid height for S. tundrensis. GS: Gamssulzen, NL: Nixloch, SF: Sesselfelsgrotte, rec: recent sampling (this study), Pl: plain, Mn: mountains, Fh: foothills, Tun: tundra.