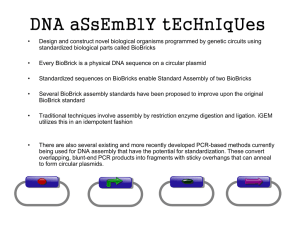
methods-plasmids
advertisement

Plasmids ZNF Army pCAG EV. pCAG EN (CEPKO Lab) was ordered (Addgene plasmid 11160) that contained multiple cloning sites for subcloning. pCAG GFP. pCAG GFP (Cepko Lab) was ordered (Addgene plasmid 11150) and used as a transfection efficiency control as well as a co-transfection plasmid fort FACS applications in the Tc11 Rescue experiments. pCAG ZNF91. ZNF91 cDNA sequence was synthesized as a codon-optimized pUC57 insert flanked by EcoRV sites from Genscript. This insert was EcoRV digested and inserted into the pCAG EN vector at EcoRV. pCAG ZNF91 HA. pCAG ZNF91 was used as a starting source to add a C-terminal HA tag via PCR for Co-IP and western blot analysis. pCAG ZNF33a. IMAGE clone 8991835 was ordered from ATCC and PCR amplified and ligated into pENTR/D/TOPO and moved to pCAG-DEST via LR clonase reaction. pCAG ZNF90. IMAGE clone 9021208 was ordered from ATCC and PCR amplified with a C-terminal HA tag into pENTR/D/TOPO and moved to pCAG-DEST via LR clonase reaction. pCAG ZNF93. cDNA sequence was synthesized as a codon-optimized pUC57 insert flanked by EcoRV sites from Genscript. This insert was EcoRV digested and inserted into the pCAG EN vector at EcoRV. pCAG ZNF93serF HA. ZNF93 Human with the 4 contact residues substituted to Ser at fingers 813 as described(Moore, Choo, & Klug, 2001) thanks to personal communication with Prof. Dave Segal (UC Davis Genome Center). cDNA sequence was synthesized as a codon-optimized pUC57 insert flanked by EcoRI/NotI sites from Genscript. This insert was EcoRI/NotI digested and inserted into the pCAG EN vector at EcoRI/NotI. pCAG ZNF254. IMAGE clone 5296940 was ordered from ATCC and PCR amplified. This was ligated into pENTR/D/TOPO and moved to pCAG-DEST with LR reaction. znf254_ENTR_DG_F-CAC CTG TTA CCA GCA GGT ATT GGA GAT CC znf254_ENTR_DG_R-GCG CCT CTC TCA GGT TTG TAG TTT C pCAG ZNF443. IMAGE 5583971 was ordered from ATCC and using restriction digests was moved form pSPORT6 to pCAG. pCAG ZNF460. hESC cDNA was used to PCR amplify ZNF460 and this was ligated to pENTR/D/TOPO. This was moved to pCAG-DEST with LR reaction. pCAG ZNF486. IMAGE clone 40125819 was ordered from ATCC and PCR amplified with HA C-terminal primer from pCR4TOPO. This was ligated into pENTR/D/TOPO and moved to pCAG-DEST with LR reaction. pCAG ZNF519. IMAGE clone 4806717 was ordered form ATCC and moved from pDNRLIB using restriction enzymes to pCAG. pCAG ZNF544. IMAGE clone 6199648 in pSPORT6 was ordered from ATCC and using restriction enzymes was moved to pCAG. pCAG ZNF587. IMAGE clone 8991953 was ordered through Thermo. This was PCR cloned from the IMAGE plasmid. The amplicon was ligated to pENTR/D/TOPO and subsequently moved to pCAG-DEST with LR clonase. F- caccCCGTGACGGCGACCACTG R- GCTACTCAGGAGGCTGAGGTAGGAG pCAG ZNF589. IMAGE clone 100066351 was ordered through Thermo. This was PCR cloned from the IMAGE plasmid. The amplicon was ligated to pENTR/D/TOPO and subsequently moved to pCAG-DEST with LR clonase. pCAG ZNF714. IMAGE clone 4797729 was ordered through ATCC. This was PCR cloned from pBluescript clone using primers below. This amplicon was ligated to pENTR/D/TOPO and subsequently moved to pCAG-DEST with LR clonase. This clone represents unitprot isoform 3 (Q96N38-3). ZNF714-F caccCTAGAAATGGGCGACCTGAG, ZNF714-R AGACACCACACCTGGCCTAC pCAG ZNF721. cDNA sequence was synthesized as a codon-optimized pUC57 insert flanked from Genscript. This insert was moved to the pCAG EN vector at EcoRV. Domain deletion analysis of ZNF91 pCAG ZNF91 [1-11]. pCAG ZNF91 was digested with XmaI and NotI and religated resulting in the removal of the C-terminal 12-36 domains. pCAG ZNF91 [1-11] HA. pCAG ZNF91 [1-11] was used as a starting source to add a C-terminal HA tag via PCR and a N-terminal CACC site for ligation into pENTR/D/TOPO (Invitrogen). This was used in an LR reaction to transfer the insert to pCAG-DEST. This construct was used for western blot validation of ZNF91 [1-11]. pCAG ZNF91 [1-24]. pCAG ZNF91 was digested with AleI and NotI and religated resulting in the removal of the C-terminal 25-36 domains. pCAG ZNF91 [1-30] .pCAG ZNF91 was digested with NsiI and NotI and religated resulting in the removal of the C-terminal 31-36 domains. pCAG ZNF91 [1-2; 23-36.]. SacI. present in the gene-optimized synthesized ZNF91 (Genscript) after it was ligated into pCAG. RECONstruction ZNF91 and ZNF93 Ancestral proteins pCAG ZNF91hominine HA. ZNF91 representing the LCA of HCG cDNA sequence was synthesized as a codon-optimized C-terminal HA pUC57 insert flanked by EcoRI and NotI sites from Genscript. This insert was EcoRI and NotI digested and inserted into the pCAG EN vector at the EcoRI NotI. pCAG ZNF91great ape HA. ZNF91 representing the LCA of HCGO cDNA sequence was synthesized as a codon-optimized C-terminal HA pUC57 insert flanked by EcoRI and NotI sites from Genscript. This insert was EcoRI and NotI digested and inserted into the pCAG EN vector at the EcoRI NotI. pCAG ZNF91macaque HA. ZNF91 macaque cDNA sequence was synthesized as a codonoptimized C-terminal HA pUC57 insert flanked by EcoRV sites from Genscript. This insert was EcoRV digested and inserted into the pCAG EN vector at the EcoRV. pCAG ZNF93 Human. pCAG ZNF93great ape. ZNF93 representing the LCA of HCGO cDNA sequence was synthesized as a codon-optimized C-terminal HA pUC57 insert flanked by EcoRI and NotI sites from Genscript. This insert was EcoRI and NotI digested and inserted into the pCAG EN vector at the EcoRI NotI. pCAG ZNF93macaque. ZNF93 representing the macaque cDNA sequence was synthesized as a codon-optimized C-terminal HA pUC57 insert flanked by EcoRI and NotI sites from Genscript. This insert was EcoRI and NotI digested and inserted into the pCAG EN vector at the EcoRI NotI. Luciferase Reporter constructs pGL4cp_SV40. This is a standard promega vector pGL4CP with SV40 minimal promoter inserted before the firefly luciferase cDNA. This plasmid was a gift from the Privalsky lab and the sequence details can be found here: http://microbiology.ucdavis.edu/privalsky/sites/default/files/plasmids/pGL4CP_reporters/ pGL4CP-SV40.txt pGL4CP-SV40-DEST. An rfB cassette (Invitrogen) was introduced at a blunted BglII upstream of SV40 in pGL4CP-SV40 to give a GATEYWAY compatible destination vector(Onodera et al., 2012) to screen KAP1+ repeat sequences. pGL4cp-OCT4Enh-SV40. ~1500bp OCT4 human enhancer region was PCR cloned from hESC gDNA with primers and ligated upstream of SV40 at the EcoRI blunted site. OCT4Enh F-CACCCCCTCCACTATGGAACCTGCAC, OCT4Enh R- AGCTGGCCTTGGCTGAAGTG >OCT4Enh CCCTCCACTATGGAACCTGCACATCAGGTTCCTTGCTCCCCTCTCAACCAAAACTC AGACATCTAATACCACGGTAGGCCCCGTTCTCCCTCCCCCACCTCCCTGGCCCAG GCCTCCAGCCCTAGGCCCTGGGTGGGGAAAACCAGGGGGTGGGGGGTGTGGAG AAAAAATATCTGACTTCAGGTTCAAAGAAGCCTGGGAGGGACTGGGGGAAGGGGG CAGGACAATGGCCTTGGCTGGACAATCCCGGTCCCCAGAGGGGGCAGCTCTAAC CCTAAACAAGTGCTCAACCCTTGAATGGGCCTGGATGGCTCCCCTGGGGACTGCT TCCTGCTCCCCAACCCCCCAGTCCCAATCCCCTCACACAGAATCCCCTTCAGAGAC GCTAAAAGGAGCTCCAGCAACCCCCCTCTGCAATCCCCTCAAAGACTGAGCCTCA GACGGGCACCAAGGGCCCCCCACAGGGACCTAGGTATCTAGTTCCTCCTTCCTCT GGGGGACTCAGGCGTCCAGCTTCATCGTGCGTCCCTCCCCGAGCCTGGCAGATT GAGGGATGTGCTTTGTTTAGTGGGGCTGGCTGGCAGAAAGACGCAGAGGAGGTG GAGAGTGATTTGTGGAGGCGTGCAGGAAGGCTGCCCTAAGCTCCCCTTCAGGGTC TGTTTTTCTGGGCCTGGCCTGAGTATCCTGAGGCTCATGCTGCTGGTCTAGTGCTT GATTCTGTTTGCAAGAGAATAGCCAACGGAATGCCTGTCTGTGAGGGATGATGTTT GTCTGTCTGCTCCCAAAACTTGATCTCAGTGGAGGGCCTGGGGTAAGTCTGGGGG CTCCAGAGGGGGCTCTGGGCCAGGGCTCCCCACAGCTTCGAAGGCCAGAAGGCC AGGTCTGGACTGGGCACGCTGACCTCTGTCGACTTAAGTAAGGCTTCTCATTGCAG GCTCCAGGCTCAGCCCTGCCTGGGCTTGTCTGCTGAGGTCAGTGGCTCTATCTGC CTTCTAAGGGGATGGGTGTCCCGTGGCCAGCTGTCTTCATCTTGGTGGCATCCGT GAGTCTTTTGAGACTTTTCCCCCACTCTTATGTTGCCTCTGTTCGTGTGCCCATCTC CTGTCTGTGTAGACTTTTTGAGCCTAATTGTATGCGTGCATTTCAATACCTGCCACA GGTCTGCCGGAAGGTCTACAAGGCAGTGGGGTTGCAGCTGTGTTCACTTCTCGGC CTTTAACTGCCCAAAAGGCAGGTAGATTATGGGGCCTGGTGGGGGTAGGAGGAAC ATGCTTCGGAACAGGAGGAGGCCCCTCCCCAGCCATCTCAATCCCCAGGACAGAA CCATCACGGCACCTTTGTCATGCATCTCTCTGCTGTCTGCCAAGAAGACGGCCTCT CAGAGGAGGGGGAGGGGCAGGCCTGGGATTTGGCTGGAATCTCCACACCAGTGT TTCTCAGCTTGCCATCCTCCAGGTTCCCCAAAAGCGCTCTTCCCAAGCCAGTCCAG AGAGTCCCTGCTGCCCATTTTCCTAGTGGCTCCTAAAACACCTTCCCCAATTTCCC CACTCAACACCACCCTCTTGTTTTTAGATTATAATTTGTACTGTAGGTGGTGTATTTC TGGCCTGGGCAAGAGGCCCATTCCCGAGAGGGACGCAGACAAGGGGTGGGTGC CTGGGTCCCTGGCTGCCTTGTGGCTGGATATGAGCCCAGTCAGGGGTCAGCCTCC TGCATGCCTAGACTCCTAGCCGGCCCCCTTCTGGGGTGCTCAGGGCTGATGGGA GGTTGAGGCAGGCTTTCCTTCCTTCTCACTGTCCTGTTATGCCTGAAGGGTAGGTG GCTTCACTTCAGCCAAGGCCAGCT pRL-TK. This is a standard promega vector for Renilla with TK minimal promoter inserted before the renilla luciferase cDNA(Onodera et al., 2012) used as a transfection efficiency control at a ratio of 10 firefly : 1 renilla to normalize the firefly luciferase activity in each well (ff/ren). pGL4cp-SINE-R-SV40. SINE-R domain from SVA_D full length (~XcmI site through polyA) was synthesized as a gBlock (IDT) and ligated into pENTR/D/TOPO (Invitrogen). This was moved to pGL4CP-SV40-DEST with the LR GATEWAY reaction. pGL4cp-SVA_D-SV40 (full length). Synthesized pUC57-SVA_D (Genescript) was EcoRV digested and ligated directly to pGL4CP-SV40 at a blunted EcoRI, upstream of SV40. > SVA_D-SV40 (full length) TCTCCCTCTCCCTCTCTTTCCACGGTCTCCCTCTCCCTCTCTTTCCACGGTCTCCCT CTCCCTCTCTTTCCACGGTCTTCCTCTGATGCCGAGCTGAAGCTGGACTGTACTGC TGCCATCTCGGCTCACTGCAACCTCCCTGCCTGATTCTCCTGCCTCAGCCTGCCGA GTGCCTGCGATTGCAGGCGCGCACTGCCACGCCTGACTGGTTTTCGTATTTTTTTG GTGGAGACAGGGTTTTGCTGTGTTGGCCGGGCTGGTCTCCAGCTCCTAACCGCGA GTGATCCGCCAGCCTCGGCCTCCCGAAGTGCTGGGATTGCAGACAGAGTCTCGTT CACTCAGTGCTCAATGGTGCCCAGGCTGGAGTGCAGTGGCCTGATCTCGGCTCGC TACAACCTCCACCTCCCAGCCGCCTGCCTTGGCCTCCCAAAGTGCCGAGATTGCA GCCTCTGCCCGGCTGCCACGCCATCTGGGAAGTGAGGAGCGTCTCTGCCTGGCC GCCCATCGTCTGGGATGTGAGGAGCCCCTCTGCCTGGCTGCCCAGTCTGGGAAG TGAGGAGCGCCTCTTCCCAGCCGCCATCCCATCTAGGAAGTGAGGAGCGTCTCTG CCCGGCTGCCCATCGTCTGAGATGTGGGGAGCGCCGCCGCCCTGTCTGGGACTT GAGGAGCGCCTCTGCCCGGCCGCCCTGTCTGGGATGTGAGGAGCGCCTCTGCCC GGCCGTGACCCTGTCTGGGAGGTGAGGAGCGTCTCTGCCCGGCCGCCCCGTCTG AGAAGTGAGGAGCCCCTCCCCCCAGCAGCCGCCCCGTCTGAGAAGTGAGGAGCC CCTCCGCCCGGCAGCCACCCGATCTGGGAAGTGAGGAGCGTCTCCGCCCGGCAG CCGCCCCGTCCGGGAGGGAGGTGGGGGGTCAGCCCCCGCCCGGCCAGCCGCC CCGTCCGGGAGGTGGGGGCGCCTCTGCCCGGCCACCCCTTCTGGGAAGTGAGG AGCCCCTCTGCCCGGCCACCACCCCGTCTGGAAGGTGTACCCAACAGCTCACTGA GAACGGGCCATGATGACAATGGCGGTTTTGTGGAATAGAAAAGGGGGAAAGGTGG GGAAAAGATTGAGAAATCGGATGGTTTCTGTGTCTGTGTAGAAAGAAGTAGACATG GGAGACTTTTCATTTTGTTCTGTACTAAGAAAAATTCTTCTGCCTTGGGATCCTGTT GATCTATGACCTTACCCCCAACCCTGTGCTCTCTGAAACATGTGCTGTGTCCACTC AGGGTTAAATGGATTAAGGGCGGTGCAAGATGTGCTTTGTTAAACAGATGGTCGAA GGCAGCATGCTCGTTAAGAGTCATCACCACTCCCTAATCTCAAGTACCCAGGGACA CAAACACTGCGGAAGGCCACAGGGTCCTCTGCCTAGGAAAACCAGAGACCTTTGT TCACTTGTTTATCTGCTGACCTTCCCTCCACTATTGTCCTATGACCCTGCCAAATCC CCTCTGCGAGAAACACCCAAGAATGATCAATAAAAAAAAAAAAGAAAAGAA no hex. pUC57 SVA_D was digested with EcoRV/BglI resulting in ‘ChopD.2’ a fragment was 1419bp fragment lacking the Hexamer (CCCTCTn)domain. This was ligated into pGL4CP-SV40, upstream of the SV40 promoter. no hex/Alu. pUC57 SVA_D was digested with EcoRV/EagI resulting in ‘ChopC.1’ a 787 bp fragment consisting of the 3’ side of SVA and was ligated into pGL4CP-SV40, upstream of the SV40 promoter. partial VNTR. pGL4cp-SVA_D-SV40 (full length) was digested with Tth111I which cuts twice liberating a 401bp fragment, and leaving 1136bp of the SVA_D which was self ligated as ‘VNTR delta C’ no VNTR. ‘E’. pGL4cp-SVA_D-SV40 (full length) was digested with Tth111I/XcmI which removes 401bp+321bp fragments, and leaving 813bp of the SVA_D which was self ligated as ‘VNTR delta D’. no SINE-R. pUC57 SVA_D was digested with EcoRV/EagI resulting in ‘Chop C.3’ a s 686bp 5’ SVA fragment. This was ligated into pGL4CP-SV40, upstream of the SV40 promoter. partial Sine-R. pGL4cp-SVA_D-SV40 (full length) was digested with PpuMI/XcmI which removes a 350bp fragment, and leaving 1187bp of the SVA_D which was self ligated as VNTR delta A. Alu-VNTR (1-1.5). pUC57-SVA_D was digested with PfIMI/Tth111I generating a 180bp Alu domain and VNTR domain containing fragment including 63bp of the VNTR domain or about 1.5 VNTR repeat units. This was cloned upstream of OCT4Enh at EcoRI blunt in pGL4cp-OCT4Enh-SV40 as ‘VNTR OCT4Enh F'6’. VNTR (8.5-15). pUC57-SVA_D was digested with Tth111I/XcmI generating a 323bp VNTR domain containing fragment or about 6.5 VNTR repeat units. This was cloned upstream of OCT4Enh at EcoRI blunt in pGL4cp-OCT4Enh-SV40 as ‘VNTR OCT4Enh D3’. VNTR (1.5-8.5). pUC57-SVA_D was digested with Tth111I/PfIMI generating a 220bp VNTR domain containing fragment or about 7 VNTR repeat units. This was cloned upstream of OCT4Enh at EcoRI blunt in pGL4cp-OCT4Enh-SV40 as ‘VNTR OCT4Enh F4’. Alu-VNTR (1-7.5). pUC57-SVA_D was digested with Tth111I generating a 401bp Alu and VNTR domain containing fragment or about 7.5 VNTR repeat units. This was cloned upstream of OCT4Enh at EcoRI blunt in pGL4cp-OCT4Enh-SV40 as ‘VNTR OCT4Enh C6’. VNTR (1.5-15). pUC57-SVA_D was digested PfImI/XcmI with generating a 544bp VNTR domain containing fragment or about 13.5 VNTR repeat units. This was cloned upstream of OCT4Enh at EcoRI blunt in pGL4cp-OCT4Enh-SV40 as ‘VNTR OCT4Enh E2’. pGL4cp-L1PA4-5’UTR-SV40. Synthesized ~500bp L1PA4 (gBLOCk, IDT) was cloned into pENTR-D/TOPO (Invitrogen) and transferred by GATEWAY reaction to pGL4CPSV40-DEST. > L1PA4-5’UTR GGGGGAGGAGCCAAGATGGCCGAATAGGAACAGCTCCGGTCTACAGCTCCCAGC GTGAGTGACACAAAAGACGGGTGATTTCTGCATTTCCAACTGAGCTTTGAAGAGAG TAGTGGTTCTTCCAGCATGCAGCTTGAGATCTGAGAACAGGCAGACTGCCTCCTCA AGTGGGTCCCTTACCCCCGAGTAGCCTAACTAGGAGGCACCCCCGAGTAGGGGC AGACTGACACCTCACATGGCAGGGTACTCCTCTGAGACAAAACTTCCAGAGGAAC GATCAGGCAGCAGCATCTGCAGTTCACCAATATCTGCTGTTCTGCAGCCACTGCTG CTGATACCCAGGCAAACAGGGTCTGGAGTGGACCTCTAGCAAACTCCAACAGACC TGCAGCTGAGGGTCCTGTCTGTTAGAAGGAAAACTAACAAACAGAAAGGACATCCA CACCAAAACCCACCTGTAC 129L1PA4. Synthesized 129bp L1PA4 (gBLOCk, IDT) with flanking sequence from L1HS at the site of deletion (PvuII sites) was cloned into pENTR-D/TOPO (Invitrogen). This was next PvuII digested and ligated into pGL4cp-OCT4Enh-SV40 at EcoRI blunt upstream of OCT4Enh and SV40 promoter. 129scramble. Same as 129L1PA4 except the 129bp element was scrambled before being syntheized as a gBlock. 51L1PA4. Same as 129L1PA4 except a 51bp region, beginning at the Persikov predicted 18mer/ Zn Finger domains 8-13 was chosen as the anchor point such that 5’-3’ DNA would be bound by a C-N terminal oriented protein where each finger binds to 3bp. 12951L1PA4. Same as 129L1PA4 except a 51bp region in 51L1PA4 was deleted from the 129bp element at the gBlock synthesis step. Retrotransposition reporter constructs L1HS. A gift from the Kazazian lab (JHMI). Thanks to Dustin Hancks for sending plasmids plasmid map thanks to John Goodier. Hancks description: 99 RPS EGFP- hot L1, L1-RP (Kimberland et al 1999), cloned into pCEP4 (Invitrogen) lacking the CMV promoter (Moran et al 1996), L1 is driven by its own promoter in the 5’-UTR, puro selection, digest with NotI/Bstz17I, two bands 6kb (the L1) and 11 kb backbone + EGFP retrotransposition indicator cassette, (Ostertag et al 2000 NAR). L1HS+129bp. L1HS plasmid was Digested with NotI/Bstz17I and ligated to pCAG EN at NotI/EcoRV. Next, pCAG-L1HS was digested with PvuII, and an insert from the PvuII fragment from 129L1PA4 was ligated. Finally, pCAG-L1HS-129L1PA4 was digested with NotI/AleI and ligated back to the pCEP4 based vector that originally contained L1HS insert. This final plasmid is meant to represent the L1PA4/3 ancestral state. L1HS+129 scr. Same as L1HS+129bp, except the 129L1PA4 fragment is the 129scramble insert. SVA_D and L1PA4 domain analysis was performed with a combination of restriction enzymes and gBLOCK synthesized fragments. VNTR-OCT4Enh-pGL4CP-SV40 was created by removing a 545bp, 13.5 repeatcontaining VNTR subunit fragment between PflMI and XcmI. Selected KRAB ZNFs were obtained from cDNA, synthesized by Genscript, or subcloned from I.M.A.G.E. vector through ATCC and ligated into pCAG_EN (Cepko Lab, Addgene) either directly at EcoRI blunt site or through Gateway ENTRY cloning using pCAG_EN with an rfA cassette integrated at the EcoRI site.