Use the terms in the boxes to fill in the blanks in the following
advertisement

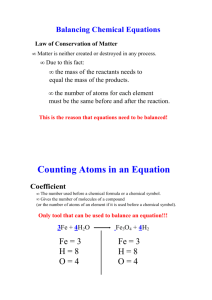
Use the terms in the boxes to fill in the blanks in the following paragraphs. Atoms Correct Mass Balanced Element Mg Chemical Reaction Equation Numbers Coefficients Formula Products Reactants Substance Two Yields A ___________________________ is a well defined example of a chemical change. A chemical ___________________________ can be used to show the changes that occur in a chemical reaction. In a chemical reaction, the substance on the left side of the arrow are the starting substance. These substances are called ___________________________. The substances on the right side of the arrow are the substances that result from the reaction. These substances are called ___________________________. The arrow is read as either produces or ___________________________. According to the law of conservation of ___________________________, atoms are neither lost nor gained during a chemical reaction. This law is illustrated when a chemical equation is ___________________. When this is done, there will be the same number of ___________________________ of each kind on both sides of the equation. In a chemical equation, the numbers that are placed in front of the symbols and the formulas are called ___________________________. They are necessary to keep the ___________________________ of atoms in balance. There are several rules for balancing an equation. First, write the correct _________________________ for each reactant and product. Next, choose the coefficients that make the number of atoms of each ___________________________ on each side of the equation equal. The correctly written formula should not be changed. If you change the formula of a substance, the equation is no longer ________________________. Changing a formula will indicate a ___________________________ different than the one intended. To balance the equation Mg + O2 MgO, first choose coefficients to make the number of atoms of each element on each side of the equation equal. You would need to place a coefficient of ___________________________ in front of the product MgO. You would also need to place a coefficient of two in front of the reactant ___________________________. Use the equations to answer the questions. Zn + S ZnS 1. What are the reactant(s) in this chemical equation? __________________________________________ 2. What are the product(s) in this chemical equation? __________________________________________ 3. According to the law of conservation of mass, if the total mass of the product in this chemical reaction is 14 grams, what must be the combined masses of the reactants be? ______________________________ 2H2 + O2 2H2O 4. What are the reactant(s) in this chemical equation? __________________________________________ 5. What are the product(s) in this chemical equation? __________________________________________ 6. What do the coefficients tell you about the ratio of reactants? _________________________________ __________________________________________________________________________________ 7. How many units of product are produced? ________________________________________________ Write the chemical equations for the following reactions. 8. Two units of sodium plus one unit of chlorine gas produce two units of sodium chloride. __________________________________________________________________________________ 9. One unit of methane (CH4), plus two units of oxygen (O2) produce one unit of carbon dioxide (CO2) and two units of water. ___________________________________________________________________ 10. One unit of aluminum sulfate plus three units of barium chloride yield two units of aluminum chloride plus three units of barium sulfate. _______________________________________________________ Label the parts of the equation using the following words: Coefficient, Reactants, Products, Subscripts, Yields. 2 K + MgBr2 2KBr + Mg Classify the following chemical reactions as: Synthesis, Decomposition, Single Displacement, or Double Displacement. _______________________________________ 2H2 + O2 2H2O _______________________________________ 2H2O 2H2 + O2 _______________________________________ Zn + H2SO4 ZnSO4 + H2 _______________________________________ 2CO + O2 2CO2 _______________________________________ 2HgO 2Hg + O2 _______________________________________ 2KBr + Cl 2 2KCl + Br2 _______________________________________ CaO + H2O Ca(OH)2 _______________________________________ AgNO3 + NaCl AgCl + NaNO3 _______________________________________ 2H2O2 2H2O + O2 _______________________________________ Ca(OH)2 + H2SO4 CaSO4 + 2H2O True/False. Write out the word “true” or “false” for each statement. ____________________The new substances formed as a result of a chemical reaction are called products. ____________________The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. ____________________A chemical equation must be balanced in order to show that mass is conserved during a reaction. ____________________Because the equation N2H4 + O2N2+H2O has two nitrogen atoms on each side, the equation is balanced. ____________________A decomposition reaction is the opposite of a synthesis reaction. ____________________A combustion reaction is a reaction in which a substance reacts with carbon dioxide, often producing heat and light. ____________________The reaction that forms water can be classified as either a synthesis reaction or a combustion reaction. ____________________The formation of chemical bonds absorbs energy. ____________________In exothermic reaction, the energy required to break the bonds in the reactants is greater than the energy released as the products form.