Enzyme
advertisement

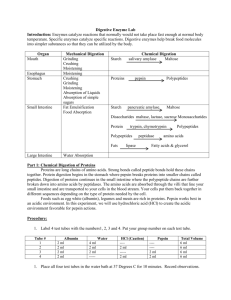
Name: _____________________________________ Per: ______ ENZYME- LAB BACKGROUND Enzymes are molecules that belong to the _________________________ macromolecule group. They catalyze Rx which means they ____________ up the rate of a chemical reactions but are not ________________________during the reaction. At the end of the reaction catalysts can be recovered in their original form. Each metabolic Rx in the body requires its own specific enzyme, because each enzyme fits only one specific substrate a phenomenon that is commonly referred to as the ___________________ mechanism. For example the function of amylase enzymes in our saliva and pancreatic secretions is to hydrolyze the starch in our diets. Other examples of enzymes are __________________ which break the esterbonds of triglycerides or the enzyme _____________________ which breaks the bond between glucose and fructose in the ____ saccharide sucrose. The three-dimensional tertiary structure of the protein plays a very important role in the function of enzymes. The tertiary/quaternary structure helps maintain the shape of the protein such that the protein is able to recognize and bind a select group of molecules called ____________________ The region of the enzyme responsible for binding the substrate and carrying out the reaction is called the ___________________. The active site of an enzyme has a specific three-dimensional shape that allows only one or only a small number of substrate molecules to bind, making enzymes highly specific. An enzyme with a substrate bound to the active site is called an ______________________________. The activity of the enzyme, or how fast the enzyme works, is strongly influenced by reaction conditions such as the enzyme concentration, temperature, pH and the presence of inhibitors. Most enzymes in the body function optimally physiological conditions: pH of 7.4, temperature of 38 Celcius (98.4 0F) Under these conditions, the enzyme has the perfect _________________________--shape to bind the substrate. In this lab you will test the activity of the enzyme Amylase. Comlete the reaction below by indentifying the substrate and the product of Amylase Amylase Testing for enzyme activity In order to test for enzyme activity, you need to be able to measure concentrations of substrate and product. What is the test for starch and how does a positive and negative test look like? What is the test for glucose and how does a positive and negative test for glucose looks like? Enzyme Concentration: The rate of an enzyme reaction also depends on the enzyme concentration, the _________ enzyme molecules are present the _______ the reaction goes. Temperature: The temperature at which an enzyme is most active is called the optimum temperature. Above and below the optimum temperature the activity of the enzyme is reduced. Most enzymes in the human body work best at around the temperature of ________________ Some types of bacteria that are found near geothermal vents, where the temperature is significantly higher and therefore have enzymes that function optimally in these ranges. If the 1 Name: _____________________________________ Per: ______ enzymes experiences temperatures significantly higher than its optimal temperature, the enzyme _________________ which means it starts to break down. pH: Enzymes are most active at their optimum pH. At pH values above or below the optimum pH the activity of the enzyme is reduced. Most cellular enzymes have an optimum pH close to the physiological pH of 7.4. In contrast enzymes that work in the stomach have optimum values around pH 1 at which they obtain a perfect 3D structure. Interesting enough the stomach is the part of the digestive system to digest exclusively the proteins found in our food. The acid __________________ the food proteins which is the first step of breaking them down Inhibitors: Molecules that prevent the active site from binding the substrate and cause enzymes to lose activity are called inhibitors. Inhibitors that compete with the substrate for the active site are called ____________________________ inhibitor. Inhibitors that bind to a site other than the active site, but still changes the active site conformation and prevents substrate binding are called a _______-competitive inhibitors. PROCEDURE The goal of this laboratory is to introduce you to some of the concepts relating to the activity of enzymes. You will observe how enzyme concentration, temperature, pH and inhibitors affect the activity of the enzyme amylase. Amylase is present in our saliva as well as our pancreatic secretions and is responsible for hydrolyzing the carbohydrate amylose (a component of starch) in our diet. Amylase will hydrolyze amylose to give smaller polysaccharides, disaccharides such as maltose, and given sufficient time the monosaccharide glucose. Eventually amylase will convert all the amylose to glucose. You will determine the activity of amylase by testing for the presence of amylose in a sample of starch using iodine. Iodine produces a deep blue-black color with amylose. As the amylase enzymes breaks down the amylose in the starch the iodine test will become less and less positive. When all of the amylose has been converted to glucose the iodine test will be negative and only the red or gold color of the iodine reagent will be seen. Based on the color with the iodine reagent and how fast the blue-black color is lost you can determine the activity of amylase. You will also use the Benedict’s test to detect the appearance of the hydrolyzed product, glucose. You will use the iodine test to investigate how enzyme concentration, temperature, pH and an inhibitor affect the activity of amylase. Constant temperature water baths. Type of Bath Temperature of Water Bath Beaker with Boiling hot water 100°C Adjustable Water bath 37°C Beaker with Ice water 0°C (or lower than 5°C) Starch solution. Place 20 mL of 1% starch solution in a 50 mL beaker to use as needed Iodine solution. Use iodine dropper bottles. Amylase. Store 10 mL of amylase solution provided by the instructor in a Styrofoam ice box. Reference test. Place 5-6 drops of starch solution in an empty well of your plate and add 1-2 drops of iodine reagent. The reaction with starch should give a deep blue-black color. 2 Visual Color Reference. As you proceed with each experiment, you will check enzyme activity by adding iodine to the starch mixture. When enzyme activity is high, the time required for the starch to hydrolyze will be very short. When the enzyme is slowed down or inactive, the blueblack color will be seen for a longer time. By observing the disappearance of starch, you can assess the relative amount of enzyme activity as follows: Iodine Test Color Amount Starch Remaining Enzyme Activity Level All None (0) Gray/Brown Most Low (1) Light brown Some Moderate (2) Gold None High (3) Dark blue black Use clean pipettes when transferring solutions to avoid cross-contaminating samples. PROCEDURE A: EFFECT OF ENZYME CONCENTRATION, CONSTANT TEMPERATURE AT 37 C Materials: Micro-centrifuge tubes, 37°C water bath, 400 mL beaker, floating rack, plastic 24 well plate, amylase solution, pipettes, 1% starch (buffered pH 7.0), and iodine reagent. 1. 2. 3. 4. Place 1 mL of 1% starch solution in four separate micro-centrifuge tubes labeled 1-4. Place 1.5 mL of amylase solution in a fifth tube. Place all five tubes in a 37°C water bath for 5 minutes. While the solutions are heating, prepare your well plate to check enzyme activity. Add 12 drops of iodine solution to 4 empty wells in 3 adjacent rows (3x 4=12) 5. At the end of the 5 minute warming period add the respective drops of warmed amylase (Table below!) from tube 5 to the labeled 4 starch containing tubes. Cap tube after adding the amylase and shake to mix. 6. Then immediately transfer four drops of each reaction mixture (use a clean pipette with each transfer) to the first rows of wells containing iodine reagent. Tube Number Amount 1% Starch Amount Warmed Amylase 1 1 mL 0 drops (control) 2 1 mL 3 drops 3 1 mL 6 drops 4 1 mL 10 drops RETURN THE MICRO-CENTRIFUGE TUBES IMMEDIATELY TO THE 37°C WATER BATH. AT 5 AND 10 MINUTES REMOVE 4 DROPS FROM EACH TUBE 1-4 AND REPEAT THE IODINE TEST.FOR ROW 2 AND 3. RECORD YOUR RESULTS. Record your observations for each sample. Use the visual color reference to assess the enzyme activity. Wash your well plate!!! 3 RESULT A: EFFECT OF ENZYME CONCENTRATION Observations – color produced by iodine test and activity level A.1. Time No amylase Color Level 3 drops amylase Color Level 6 drops amylase Color Level 10 drops amylase Color Level 0 min 5 min 10 min Graph A: Enzyme Concentration: Enzyme Activity at 10 minutes vs. enzyme concentration in drops. Have 3 lines for the three incubation times all in one graph. Label your axis. 4 PROCEDURE B: EFFECT OF TEMPERATURE Materials: Micro-centrifuge tubes, water baths: ice (0°C), warm (37°C) and boiling (100°C), 400 mL beaker (2), floating racks, hot plate, plastic 24 well plate, pipettes, buffer solution (pH 7), amylase solution, 1% starch, and iodine reagent. 1. Place 0.75 mL of 1% starch solution in three separate micro-centrifuge tubes. 2. Place one tube in the boiling water bath, one tube in the 37°C water bath, and one tube in the ice bath. 3. Add 6 drops of buffer solution pH 7 and 5 drops of amylase solution to three other tubes, and place one each in the three water baths. 4. Let them remain in the water baths for about 10 minutes to allow the solutions to reach bath temperature. (You should have 6 tubes, 3 containing 1% starch and three containing amylase. Each Bath should have two tubes 1 each of the starch and amylase.) For each temperature bath, proceed as follows: a. Remove the micro-centrifuge tubes, and pour the starch solution into the amylase solution, cap the tube and shake well to mix. Return the mixture to the same temperature bath and incubate for 5 min b. Add 1-2 drops of iodine reagent to 3 empty wells of row 1 in your well plate. After 5 minutes, transfer four drops of the mixture (use a clean pipette each time) to a well containing iodine reagent. Record the color and activity level. RESULTS B: EFFECT OF TEMPERATURE ON ENZYME ACTIVITY Temperature Color Level 0 0C 37 0C 100 0C Graph B: Enzyme activity at different temperatures. Label your axis. 5 PROCEDURE C. EFFECT OF PH ON ENZYME ACTIVITY Materials: Micro-centrifuge tubes, 37°C water bath, 400 mL beaker, floating rack, plastic 24 well plate, pipettes, buffers (pH 2,4, 7, and 10), amylase solution, 1% starch, and iodine reagent. 1. Place 6 drops of buffer solutions of pH 2, 4, 7, and 10 in four separate, labeled microcentrifuge tubes. 2. Add 3 drops of amylase solution in each. In four other tubes, place 0.75 mL of 1% starch solution. 3. Place all the tubes in a 37°C water bath for about 5 minutes. 4. Pour each of the 1% starch solutions into a separate buffer-amylase tube. Cap the tube and shake well to mix. Return the mixtures to the 37°C water bath. Note the time. 5. Add 1-2 drops of iodine reagent to 4 empty wells in row 2 in your well plate. After 10 minutes, remove four drops of each reaction mixture (use a clean pipette each time) and add to a well containing iodine reagent. Record your observations and the enzyme activity level for each reaction mixture. RESULTS C: EFFECT OF PH ON ENZYME ACTIVITY pH 2 4 7 10 Color Level Graph C: Enzyme activity level vs. pH. Label your axis. 6 PROCEDURE D. INHIBITION OF ENZYME ACTIVITY Materials: Micro-centrifuge tubes, 37°C water bath, 400 mL beaker, floating rack, plastic 24 well plate, pipettes, 1% AgNO3 and 1% NaCl solutions, 95% ethanol, amylase solution, 1% starch, and iodine reagent. 1. In one micro-centrifuge tube, place 3 drops of amylase solution and 10 drops of pH 7 buffer solution. 2. In a second micro-centrifuge tube, place 3 drops of amylase solution and 10 drops of 1% NaCl. 3. In a third tube, place 3 drops of amylase solution and 10 drops of ethanol. 4. In a forth tube, place 3 drops of amylase solution and 10 drops of 1% AgNO3. Label each tube. 5. Place 1 mL of 1% starch solution in four other tubes. 6. Place all eight tubes in a 37° water bath for 10 minutes. 7. Pour the 1% starch solution into the amylase solution, cap the tube and shake well to mix. 8. Return the tubes to the 37°C water bath for 15 minutes. 9. Add 1-2 drops of iodine reagent to 4 empty wells of row 3 in your well plate. 10. Remove four drops of each reaction mixture (use a clean pipette each time) and add to a well containing iodine reagent. If the AgNO3 well loses its color after transferring it to a well containing iodine reagent, add 1-2 extra drops of iodine reagent. Record your observations and the enzyme activity for each of the reaction mixtures. RESULTS D: INHIBITION OF ENZYME ACTIVITY D.1. Observations – color produced by iodine test and activity level Compound Color Level Buffer pH 7 NaCl Ethanol AgNO3 7 QUESTIONS (1). In part A you investigated the activity of amylase while increasing the enzyme concentration. Describe the effect of enzyme concentration on enzyme activity. (2). In parts B and C you investigated the activity of amylase at different temperatures and at different pH values. Based on the graphs you drew what was the optimal temperature and pH of amylase? (3). Pepsin is an enzyme that helps break down proteins in our stomach. The pH of our stomach is 2. What do you think is the optimum pH of pepsin? (4). In part D you investigated the activity of amylase in the presence of three different compounds. Which compound do you think was an inhibitor of enzyme activity? PRE-LABORATORY QUESTIONS (1). Research and draw graphs (one each) to show how temperature and pH affect the activity of an enzyme (i.e., reaction rate). Your graphs should have correctly labeled axis. On your graph indicate where you would expect to observe the optimum temperature and optimum pH for the enzyme. (2). Explain how high temperatures decrease enzyme activity as does extreme pH values 8