The Impact of a Community-Oriented Problem
advertisement
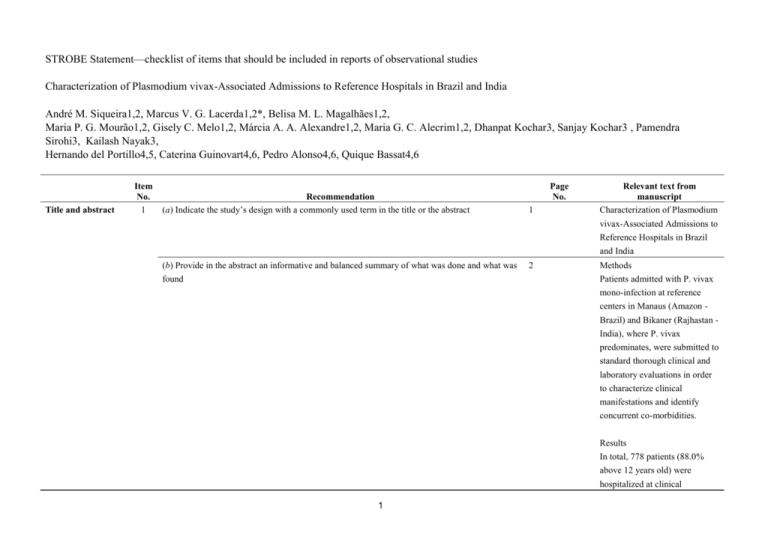
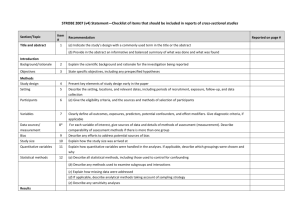
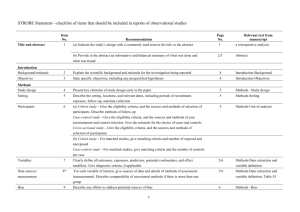
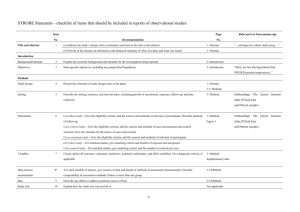
STROBE Statement—checklist of items that should be included in reports of observational studies Characterization of Plasmodium vivax-Associated Admissions to Reference Hospitals in Brazil and India André M. Siqueira1,2, Marcus V. G. Lacerda1,2*, Belisa M. L. Magalhães1,2, Maria P. G. Mourão1,2, Gisely C. Melo1,2, Márcia A. A. Alexandre1,2, Maria G. C. Alecrim1,2, Dhanpat Kochar3, Sanjay Kochar3 , Pamendra Sirohi3, Kailash Nayak3, Hernando del Portillo4,5, Caterina Guinovart4,6, Pedro Alonso4,6, Quique Bassat4,6 Title and abstract Item No. 1 Recommendation (a) Indicate the study’s design with a commonly used term in the title or the abstract Page No. 1 Relevant text from manuscript Characterization of Plasmodium vivax-Associated Admissions to Reference Hospitals in Brazil and India (b) Provide in the abstract an informative and balanced summary of what was done and what was found 2 Methods Patients admitted with P. vivax mono-infection at reference centers in Manaus (Amazon Brazil) and Bikaner (Rajhastan India), where P. vivax predominates, were submitted to standard thorough clinical and laboratory evaluations in order to characterize clinical manifestations and identify concurrent co-morbidities. Results In total, 778 patients (88.0% above 12 years old) were hospitalized at clinical 1 discretion with PCR-confirmed P. vivax mono-infection (316 in Manaus and 462 in Bikaner) of which 197 (25.3%) presented at least one severity criterion as defined by the WHO (2010). Hyperlactatemia, respiratory distress, hypoglycemia and disseminated intravascular coagulation were more frequent in Manaus. Noteworthy, pregnancy status was associated as a risk factor for severe disease (OR= 2.03; 95%CI=1.23.4; p=0.007). The overall case fatality rate was of 0.3/1000cases in Manaus and 6.1/1000cases in Bikaner, with all deaths occurring amongst patients fulfilling at least one severity criterion. Within this subgroup, case fatality rates increased respectively to 7.5% in Manaus, and 4.4% in Bikaner. Introduction Background/rationale 2 Explain the scientific background and rationale for the investigation being reported 3-5 In the absence of adequate experimental models, or the paucity of postmortem samples to further elucidate the pathophysiology of this infection [5, 13, 24], systematic 2 multisite clinical studies become a unique opportunity to better understand this geographic variability and characterize the severe vivax malaria syndrome. Objectives 3 State specific objectives, including any prespecified hypotheses 5 aiming to comprehensively characterize and compare the clinical complications of P. vivax infection. Methods Study design 4 Present key elements of study design early in the paper 7-8 This was a hospital-based study of patients admitted with vivax malaria at the two study centers, regional references mostly for the adult population. Setting 5 Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, 5-6 follow-up, and data collection The enrollment of cases was performed in two different reference tertiary hospitals located in Brazil and India. Participants 6 (a) Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants 7 All initial microscopyconfirmed P. vivax infections (or patients with negative thick blood smears [TBS] but with history of previous recent diagnosis in use of antimalarials) requiring admission, at clinician’s discretion (e.g., presence of jaundice, thrombocytopenia, bleeding, vomiting, diarrhea, abdominal pain, non-lithiasic 3 cholecystitis, spleen rupture or overall compromised clinical status), regardless of age, were eligible for enrollment provided patients or their legal representatives signed an informed consent. Variables 7 Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. 11 Give diagnostic criteria, if applicable Unadjusted simple logistic regression analyses were also performed considering different outcomes, especially the fulfilment of WHO severe criteria for P. falaciparum [20] and death Data sources/ 8* measurement For each variable of interest, give sources of data and details of methods of assessment 9-11 (measurement). Describe comparability of assessment methods if there is more than one group Patients were classified in relation to their clinical symptomatology and laboratory results Bias 9 Describe any efforts to address potential sources of bias 7 The same protocol for clinical and laboratory investigations was applied in both sites, including standard operating procedures (SOPs) and questionnaires. The study procedures were standardized and supervised by coinvestigators from both sites and supervised by study monitors from ISGlobal (Q.B. and C.G) to minimize potential 4 discrepancies in its application. Study size 10 Explain how the study size was arrived at N/A Continued on next page 5 Quantitative 11 variables Statistical Explain how quantitative variables were handled in the analyses. If applicable, describe which 11 groupings were chosen and why 12 methods (a) Describe all statistical methods, including those used to control for confounding 11 (b) Describe any methods used to examine subgroups and interactions N/A (c) Explain how missing data were addressed N/A (d) Cohort study—If applicable, explain how loss to follow-up was addressed N/A Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy Results Participants 13* (e) Describe any sensitivity analyses N/A (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined Figure 2 for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed Descriptive data 14* (b) Give reasons for non-participation at each stage Figure 2 (c) Consider use of a flow diagram Figure 2 (a) Give characteristics of study participants (eg demographic, clinical, social) and information on Table 1 exposures and potential confounders (b) Indicate number of participants with missing data for each variable of interest Table 1 & 4 (c) Cohort study—Summarise follow-up time (eg, average and total amount) Outcome data 15* Cohort study—Report numbers of outcome events or summary measures over time Case-control study—Report numbers in each exposure category, or summary measures of exposure Table 4 Cross-sectional study—Report numbers of outcome events or summary measures Main results 16 (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision Table 4 (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included (b) Report category boundaries when continuous variables were categorized Table 4 (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time Table 4 period 6 Unadjusted linear regression was undertaken to explore the association between number of severe criteria and risk factors Continued on next page 7 Other analyses 17 Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses N/A 18 Summarise key results with reference to study objectives 19 Discussion Key results the associated CFR amongst admitted patients to the study in the Brazilian Amazon (0.9%) and Indian Rajasthan (1.5%) is not negligible, Limitations 19 Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss 19 both direction and magnitude of any potential bias by not performing a systematic investigation in all admitted cases from both sites, there was an important limitation on estimating the prevalence of their occurrence and association with severe syndromes, Interpretation 20 Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of 19 analyses, results from similar studies, and other relevant evidence Our data provide yet again a robust confirmation of the potential of this species to cause significant morbidity and even mortality, a fact now widely recognized by the scientific community and by WHO in its recently published severe malaria monograph Generalisability 21 Discuss the generalisability (external validity) of the study results 19 Indeed, the associated CFR amongst admitted patients to the study in the Brazilian Amazon (0.9%) and Indian Rajasthan (1.5%) is not negligible, and comparable to CFRs previously described in Papua New Guinea [38] and Indonesia [18], and not too dissimilar to those for P. falciparum in Africa [2], although these findings must be taken cautiously as the low incidence of P. falciparum in the study areas and the hospital-based design can limit 8 comparisons and estimates of community CFRs Other information Funding 22 Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based 27 This work was mainly supported by Fundació Cellex, and also by PRONEX Malaria Network, funded by the Brazilian Ministry of Science and Technology (MCT), National Council for Scientific and Technological Development (CNPq), Brazilian Ministry of Health (DECIT/SCTIE/MS) and the Research Support Foundation of Amazonas (FAPEAM) (grant 555.666/2009-3). MVGL is a level 1 CNPq fellow and HAP is also funded by the Science without Borders (CsF), from CNPq. QB has a fellowship from the program Miguel Servet of the ISCIII (grant number: CP11/00269). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. *Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies. Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org. 9