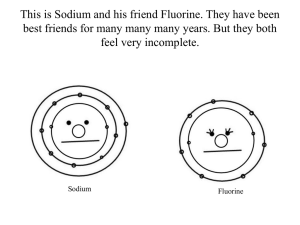
semester iii, 2013/2014
advertisement
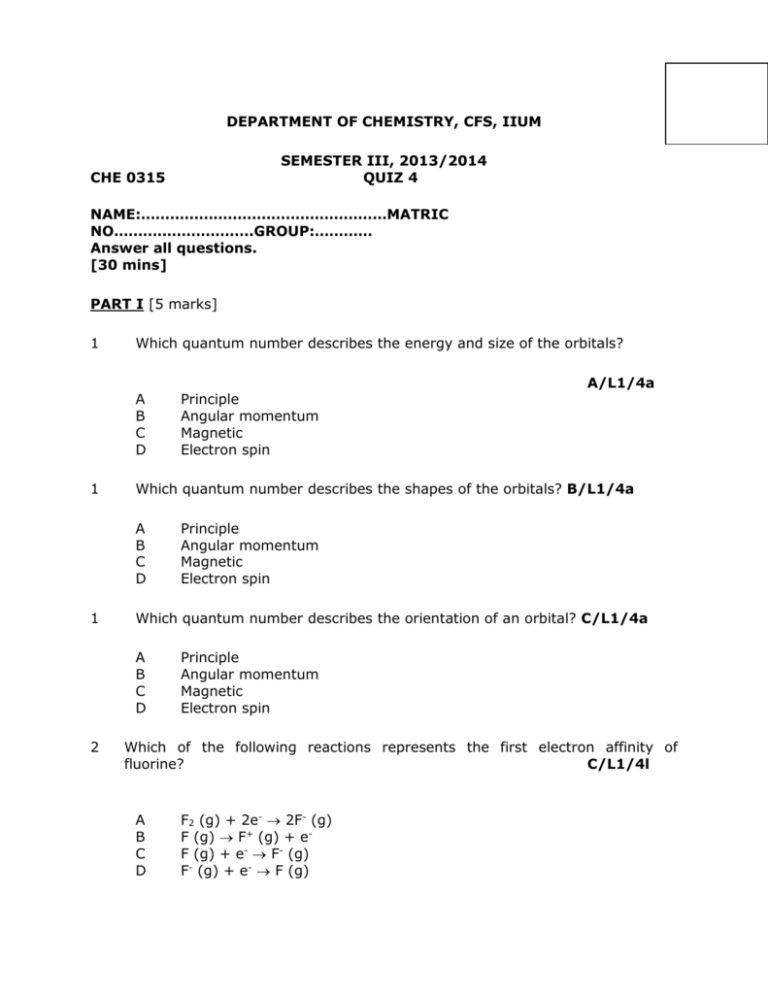
DEPARTMENT OF CHEMISTRY, CFS, IIUM SEMESTER III, 2013/2014 QUIZ 4 CHE 0315 NAME:……………………………………………MATRIC NO………………………..GROUP:………… Answer all questions. [30 mins] PART I [5 marks] 1 Which quantum number describes the energy and size of the orbitals? A B C D 1 Principle Angular momentum Magnetic Electron spin Which quantum number describes the orientation of an orbital? C/L1/4a A B C D 2 A/L1/4a Which quantum number describes the shapes of the orbitals? B/L1/4a A B C D 1 Principle Angular momentum Magnetic Electron spin Principle Angular momentum Magnetic Electron spin Which of the following reactions represents the first electron affinity of fluorine? C/L1/4l A B C D F2 (g) + 2e- 2F- (g) F (g) F+ (g) + eF (g) + e- F- (g) F- (g) + e- F (g) 2 Which of the following reactions represents the first electron affinity of chlorine? C/L1/4l A B C D 2 Cl2 (g) + 2 e- 2Cl- (g) Cl (g) Cl+ (g) + eCl (g) + e- Cl- (g) Cl- (g) + e- Cl (g) Which of the following reactions represents the first electron affinity of bromine? C/L1/4l A B C D Br2 (g) + 2 e- 2Br- (g) Br (g) Br+ (g) + eBr (g) + e- Br- (g) Br- (g) + e- Br (g) 3 Which of the following have their valence electrons in the same shell? C/L2/4e A K, As, Br B B, Si, As C N,As, Bi D He, Ne, F 3 Which of the following have their valence electrons in the same shell? D/L2/4e A Ca, Ge, Kr B Si, As,Te C Ne, Xe, I D P, Sb, Bi 3 Which of the following have their valence electrons in the same shell? B/L2/4e A Mg, P, Cl B O, Se, Te C C, P, Se D He, Ne, F 4 Which of the following is not isoelectronic with Kr? A B C D As3+ Se2Rb+ Sr2+ A/L2/4g 4 Which of the following is isoelectronic with Ar? A B C D 4 5 5 5 Fe2+ F BrCa2+ Which of the following pairs consists of isoelectronic species ? A B C D D/L2/4g A/L2/4g Cl- and K+ Mn2+ and Ar Na+ and K+ Cl- and S Which of the following represent electron configurations does not obey the Pauli exclusion principle? B / L3 /4d I II III IV [Ne] 3s1 3p5 [Ne] 3s3 3p2 [Kr] 4d12 5s2 5p3 [Ar] 3d10 4s2 4p2 A C I, II III, IV B D II, III I, IV Which of the following represent electron configurations does not obey the Pauli exclusion principle? A/ L3 /4d I II III IV [Ne] 3s2 3p7 [Kr] 4d12 5s2 5p3 [Ar] 3d10 4s2 4p5 [Ar] 3d10 4s2 4p2 A C I, II III, IV B D II, III I, IV Which of the following represent electron configurations does not obey the Pauli exclusion principle? B / L3 /4d I II III IV [Ne] 3s1 3p5 [Xe] 6s2 5d12 [Kr] 4d12 5s2 5p3 [Ar] 3d10 4s1 4p2 A C I, II III, IV B D II, III I, IV PART II [10 MARKS] [SET 1] 1. (a) Give the period number and group number for element X with the following electronic configuration; [2]/L1/4f 1s2 2s2 2p2 Period 2 & Group 14 (a) Draw all the ‘p’ orbitals occupy in the electronic configuration above [2]/L1/4b 2px 2py 2. The graph shows the five successive ionization energies in kJ/mol for element Q 40000 30000 20000 10000 0 1 2 3 4 5 (a) Identify the group number of element Q G 13 or 3A [1]/L2/4k (b) Explain your answer in (a) [2]/L2/4k Because there is a big jump between IE3 and IE4,[1] means three electron is removed from the valence shell and indicating that the element has three valence electrons.[1] (c) Write the condensed electronic configuration of Q if it is in Period 3 [1]/L2/4j [Ne] 3s2 3p1 3. Across period 2, the IE for nitrogen is higher than oxygen. Explain. [2]/L3/4i This is due to the stability of half filled 2p orbitals in nitrogen. Thus, a higher energy is required to remove the first electron[1]. Meanwhile, for the oxygen, it has an additional electron-electron repulsions in its 2p orbitals . Thus, its easier to remove the first electron. [1] [SET 2] 1. (a) Give the period number and group number for element X with the following electronic configuration. [2]/L1/4f 1s2 2s2 2p3 Period 2 & Group 15 (b) Draw all the ‘s’ orbitals occupy in the electronic configuration above [2]/L1/4b 1s 2s 2. The graph shows the five successive ionization energies in kJ/mol for element Q 25000 20000 15000 10000 5000 0 1 2 3 4 5 (d) Identify the group number of element Q G 14 or 4A [1]/L2/4k (e) Explain your answer in (a) [2]/L2/4k Because there are a big jump between IE4 and IE5,[1] means four electron is removed from the valence shell and indicating that the element has four valence electon.[1] (f) Write the condensed electronic configuration of Q if it is in Period 3 [1]/L2/4j [Ne] 3s2 3p2 4. Across period 3, the IE1 for phosphorus is higher than sulphur. Explain. [2]/L3/4i This is due to the stability of half filled 2p orbitals in phosphorus. Thus, a higher energy is required to remove the first electron[1]. Meanwhile, for the sulphur, it has an additional electron-electron repulsions in its 2p orbitals . Thus, its easier to remove the first electron.[1] [SET 3] 1. (a) Give the period number and group number for element X with the following electronic configuration. [2/L1/4f] 1s2 2s2 2p4 Period 2 & Group 16 (b) Draw all the ‘s’ orbitals occupy in the electronic configuration above [2/L1/4b] 1s 2s 1. The graph shows the five successive ionization energies in kJ/mol for element Q 16000 14000 12000 10000 8000 6000 4000 2000 0 1 2 3 4 5 (g) Identify the group number of element Q G 2 or 2A [1/L2/4k] (h) Explain your answer in (a) [2/L2/4k] Because there are a big jump between IE2 and IE3,[1] means two electron is removed from the valence shell and indicating that the element has two valence electon.[1] (i) Write the condensed electronic configuration of Q if it is in Period 4 [1/L2/4j] [Ar] 4s2 2. Across period 3, the IE1 for Magnesium is higher than Aluminium. Explain. [2/L3/4i] Mg : 1s22s22p63s2 Al: 1s22s22p63s2 3p1 [1] Removing of electron from 3p orbital is easier than 3s since it get shielded by the inner orbitals and further from nucleus, thus make the electrostatic forces become weaker.[1]