Supplementary Appendix - European Heart Journal
advertisement

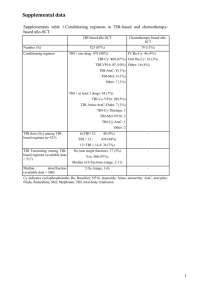
Supplementary Appendix PROTECT steering committee E Camenzind (Chairman), University of Geneva, Geneva, Switzerland L Mauri, Brigham and Women's Hospital, Boston, MA, USA W O'Neill, University of Miami Miller School of Medicine, Miami, FL, USA P W Serruys, Erasmus MC, Thoraxcentrum, Rotterdam, the Netherlands PhG Steg, INSERM U-1148, Université Paris Diderot, DHU-FIRE, AP-HP, Hôpital Bichat, Paris, France. NHLI, Royal Brompton Hospital, Imperial College, London, UK W Wijns, OLV Hospital, Aalst, Belgium Data safety monitoring board (DSMB) FWA Verheugt (Chairman), Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands ME Bertrand, Hôpital Cardiologique, Lille, France R Califf, Duke Health ORG, Durham, NC, USA D DeMets, University of Wisconsin Madison, Madison, Wisconsin, USA L Wallentin, Akademiska Sjukhuset, Uppsala, Sweden Clinical event committee (CEC) W Bocksch, Universitaitsklinikum Tubbingen, Tubbingen, Germany J Bosmans, UZA, Edegem, Belgium H Garcia, Erasmus Medisch centrum, Rotterdam, the Netherlands S Garg, Royal Blackburn Hospital, Blackburn, United Kingdom C Hanet, Cliniques Universitaires Saint-Luc, Brussels, Belgium J-PR Herrman, Onze Lieve VrouweGasthuis, Amsterdam, the Netherlands H Kelbaek, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark E Mc Fadden, Cork University Hospital, Wilton, Cork, Ireland PW Radke, Universitätsklinikum Schleswig-Holstein, Lübeck, Germany W Rutsch, Akademisches Lehrkrankenhaus der, Charité Universitätsmedizin Berlin, Berlin, Germany HH Tilsted, Aalborg Hospital, Aalborg, Denmark J Wykrzykowska, Academisch Medisch Centrum, Amsterdam, the Netherlands Independent statistician E Boersma, University Medical Center Rotterdam Erasmus, Rotterdam, the Netherlands Supplementary Figure Legend Supplmentary Figure 1. Usage of dual antiplatelet therapy at discharge, 6 months, and annually through 4 years. E-ZES=Endeavor zotarolimus-eluting stent. C-SES=Cypher sirolimus-eluting stent. Dual antiplatelet therapy defined as aspirin and a thienopyradine (clopidogrel, ticlopidine, or other). Supplementary Figure 2. Anticipated 3-year (A) and actual 4-year (B) rates of definite or probable stent thrombosis A. Anticipated 3-year rate B. Actual 4-year rate E-ZES=Endeavor zotarolimus-eluting stent. C-SES=Cypher sirolimus-eluting stent. Supplementary Table 1. List of Investigators in PROTECT Country (patients enrolled) Site Investigator Argentina (n=6) Hospital Italiano Regional del Sur C Alvarez Country (patients enrolled) Site Investigator Sanatorio Otamendi A Rodriguez Southern Health, Monash Medical Center I Meredith St Vincent's Sydney D Muller St Vincent's Melbourne R Whitbourn Royal Adelaide S Worthley Fremantle A Whelan The Prince Charles Hospital D Walters Royal Perth Hospital S Shetty Box Hill Hospital G New The Wesley Hospital S Cox Gold Coast Hospital R Batra Northern Hospital W van Gaal John Hunter Hospital G Bellamy Landesklinikum St Pölten H Mayr Salzburger Landeskliniken M Hammerer/ M Heigert Wilhelminensp der Stadt Wien K Huber AKH Linz C Steinwender/ F Leisch OLVrouwziekenhuis W Wijns UZ Leuven (Gasthuisberg) W Desmet CHR Citadelle J Boland Cliniques Universitaires UCL E Schroeder / P Chenu CHU Sart-Tilman V Legrand Ottawa Heart Institute M Labinaz London Health Sciences Center P Teefy Hôpital Laval O Bertrand Beijing Fuwai Hospital R Gao Zhongshan Hospital Fudan Univ J Ge Faculty Hospital Brno Bohunice P Kala Mas Hospital Usti nad Labem P Cervinka Dominican Republic (n=44) CEDIMAT P Ureña Finland (n=29) Kuopio University Hospital J Hartikainen Australia (n=414) Austria (n=144) Belgium (n=265) Canada (n=52) China (n=252) Czech Republic (n=19) Country (patients enrolled) Site Investigator France (n=952) AP-HP, Hôpital Bichat PG Steg Clinique Pasteur J Fajadet CHU Rangueil - Toulouse D Carrie Hôpital de la Cavale Blanche, Brest M Gilard Polyclinique des Fleurs P Barragan CHU Lille A Sudre / J-M Lablanche Clinique Saint-Hilaire, Rouen R Koning Hôpital Charles Nicolle-CHU Rouen H Eltchaninoff Clinique Saint Augustin O Darremont Polyclinique de la Louvière F Leroy CHU Michallon – Grenoble B Bertrand Clinique Saint- Pierre G Robert CHU Jean Minjoz – Besancon F Schiele Clinique Saint-Gatien, Tours S Chassaing Nouvelles Cliniques Nantaises E Bressollette / P Brunel Hôpital Trousseau – CHU L Quilliet Clinique Rhone-Durance J Brunet Hôpital Henri Duffaut M Pansieri AP-HP Hôpital Lariboisiere G Sideris / V Stratiev AP-HP, Hôpical Henri Mondor E Teiger Hôpital Pontchaillou - Rennes H Lebreton Hôpital La Timone, Marseille J-L Bonnet Clinique Saint Martin B Karsenty CH Pau N Delarche CHU Clermont Ferrand J-R Lusson / J Cassagnes Klinikum Coburg J Brachmann Universitätsklinikum Lübeck V Kurowski M Luther UnivKlin Kröllwitz M Buerke Med Hochschule Hannover A Schäfer / B Schieffer Herz- und Diabeteszentrum W Scholtz / M Wiemer Klinikum der J W Goethe Univ S Fichtlscherer / V Schächinger Germany (n=1369) Country (patients enrolled) Site Investigator Klinikum der Univ München Großhadern C Kupatt / P Boekstegers Klinikum der J Gutenberg Univ T Münzel / S Genth-Zotz Universitätsklinikum Freiburg C Bode Universitätsklinikum Heidelberg N Frey Herz Zentrum Bad Krozingen F-J Neumann Charité - Campus B Franklin C Skurk / B Witzenbichler / K Pels Herzzentrum Dresden R Strasser Asklepios Klinik St Georg K-H Kuck Krankenh der Barmh Brüder K-E Hauptmann Univ Klinikum Hamburg-Eppendorf K Sydow / S Baldus / T Heitzer Lukas Krankenhaus M Haude Klinikum Bogenhausen E Hoffmann Klinikum Villingen-Schwenningen W Jung Vivantes Klin im Friedrichshain H Ince / S Hoffmann Städtisches Klinikum Karlsruhe C Schmitt Vivantes Humboldt-Klinikum M Dissmann Klinikum Nürnberg M Pauschinger Städtische Kliniken Darmstadt G Werner University Magdeburg R Braun-Delleus Marienhof Koblenz D Burkhardt / M Manz Onassis Cardiac Surgery Center V Voudris 1st IKA D Sionis Queen Elizabeth Hospital M-L Kang-Yin Pamela Youde Nethersole Eastern Hospital T-S Tse Hungary (n=107) Semmelweis University B Merkely India (n=506) Jaslok Hospital & Res Centre A Mehta The Heart Care Clinic K Parikh Max Heart and Vascular Institute V Kumar / P Chandra Apollo Hospital, Hyderabad P Rath Ruby Hall Clinic S Hiremath St James' Hospital P Crean Greece (n=55) Hong Kong (n=59) Ireland (n=33) Country (patients enrolled) Site Investigator University Hospital Galway K Daly Rabin Med Center, Belinson Campus R Kornowski Rambam Medical Center A Kerner Meir MC M Mosseri Barzilay MC G Jafari Az Osp S Giovanni di Dio e Ruggi D'Aragona P Giudice Policlinico "A Gemelli" C Trani Ospedale S Maria Nuova A Manari Ospedale S Giovanni - Addolorata F Prati Ospedale Lancisi G Gabrielli / A Pangrazi S Donato USL 8 L Bolognese Chonnam University Hospital M-H Jeong Dong-A University Hospital M-Y Kim Seoul Nat Univ Hospital H-S Kim ASAN Medical Center S-J Park P Stradins University Hospital A Erglis Hospital "Gailezers" A Kalnins Luxembourg (n=1) INCCI D Wagner Malaysia (n=74) National Heart Institute (IJN) R Zambahari Sarawak General Hospital T-K Ong / K Sim Amphia Ziekenh Molengracht P den Heijer VU Medisch Centrum Y Appelman St Antonius Ziekenhuis M-J Suttorp Univ Med Centrum Groningen E Lipsic / B de Smet Catharina Ziekenhuis J Koolen Univ Medisch Centrum Utrecht P Stella Wellington Hospital S Harding Ascot Integrated Hospital J Warwick / A Maslowski Wakefield Hospital M Abernethy Waikato Hospital G Devlin Haukeland Universitets Sykehus S Rotevatn Israel (n=64) Italy (n=123) (South) Korea (n=254) Latvia (n=126) Netherlands (n=535) New Zealand (n=85) Norway (n=34) Country (patients enrolled) Site Investigator Feiringklinniken Y Myreng SPSK No1, ACK AMG D Ciecwierz WSS imdr WlBieganskiego J Peruga 4 Wojskowy Szpital Kliniczny K Reczuch Hospital Santa Cruz R Campante Teles Hospital Fernando Fonseca P Farto E Abreu Centro Hospital de Coimbra A Leitão-Marques Hospital Garcia Orta H Pereira Romania (n=54) Univ Hospital of Bucharest D Vinereanu Saudi Arabia (n=262) Prince Sultan Cardiac Center S Alkasab King Fahd Medical City H Mhish / M Al Kurdi King Faisal Specialist Hospital F Al Turki National Heart Center P Wong National University Hospital S-G Teo Hospital Puerta de Hierro F-J Goicolea Ruigomez Hospital Vírgen de la Arrixaca M Valdés Chávarri Hospital de Son Dureta A Bethencourt Gonzalez Hospital de Meixoeiro A Iñiguez Romo Hospital Infanta Cristina J López Minguez Hospital Clín Univ V Victoria J-M Hernández García Hospital Juan Ramón Jiménez J Diaz Fernández Hospital Univ V de la Macarena R Ruiz Salmeron Hospital Univ La Princesa A Benedicto / L Martinez Elbal Hospital Univ Marqués Valdecilla J Zueco Hospital de San Juan de Alicante RF López-Palop Hospital Virgen de las Nieves R Melgares Centrallasarettet Västerås E Diderholm / A Kåregren / O Herterich Universitetssjukhuset i Lund G Olivencrona Universitetssjukhuset Örebro O Fröbert Hôpitaux Universitaire Genève M Roffi / V Verin Centre Hospitalier Universitaire Vaudois G Girod Poland (n=86) Portugal (n=177) Singapore (n=34) Spain (n=328) Sweden (n=201) Switzerland (n=63) Country (patients enrolled) Taiwan (n=66) UK (n=1658) USA (n=178) Site Investigator Kantonsspital Aarau AG A Vuilliomenet Chang Gung Memorial Hospital LK I-C Hsieh Chang Gung Mem Hospital KS C-J Wu Glenfield Hospital A Gershlick Papworth Hospital C Densem Queen Elizabeth Medical Centre S Doshi Royal Victoria Hospital G Manoharan King's College Hospital P McCarthy James Cook University Hospital M De Belder Cardiothoracic Centre J Mills Manchester Royal Infirmary F Fath-Ordoubadi Southampton General Hospital I Simpson Leeds General Infirmary J Greenwood Cheltenham General Hospital R Chamberlain-Webber / Z Khan New Cross Hospital J Cotton City General Hospital M Gunning Morriston D Smith Royal Bournemouth S Talwar Royal Sussex County Hospital S Holmberg Freeman I Purcell University Hospital of Wales R Anderson Castle Hill Hospital S Thackray / F Alamgir Mayday Hospital N Goulielmos / K Beatt Basildon Hospital CTC P Kelly Sharp Chula Vista Med Center M Moussavian Cooper University Hospital J Aji Ocala Regional Medical Center R Prashad Dallas VA Medical Center J Hastings / A Zankar / S Banerjee Bethesda North Hospital S Lewis AnMed Health B McLaurin Emory University Hospital J Douglas Country (patients enrolled) Site Investigator Methodist Hospital S Brener Aurora St Lukes A Gupta University Hospital - Augusta L Walters Bridgeport Hospital M Driesman Baptist Hospital - Pensacola FL R Aycock Doctors Hospital at Renaissance C Mego University of Massachusetts D Fisher Maimonides Medical Center R Frankel Washington Hospital Center L Satler Supplementary Table 2A. Baseline Demographics and Patient Characteristics Characteristic E-ZES C-SES (n = 4357) (n = 4352) Age, years 62.3±10.6 62.1±10.7 0.50 Male sex 76.7 (3340/4357) 76.0 (3308/4352) 0.48 Body mass index, kg/m2 27.8±4.4 27.9±4.5 0.24 Diabetes mellitus 26.9 (1174/4357) 28.4 (1236/4352) 0.13 6.5 (285/4357) 7.4 (323/4352) 0.11 Hypertension 64.6 (2814/4357) 63.4 (2759/4352) 0.26 Hyperlipidaemia 61.9 (2695/4357) 62.8 (2734/4352) 0.35 History of smoking 57.7 (2515/4357) 57.4 (2500/4352) 0.80 24.9 (1084/4357) 25.2 (1098/4352) 0.71 Premature coronary artery disease in firstdegree relative (n = 7540) 34.2 (1288/3768) 34.8 (1312/3772) 0.59 Previous myocardial infarction 20.3 (885/4357) 20.8 (907/4352) 0.54 Previous CABG 4.6 (199/4357) 5.1 (224/4352) 0.21 Previous PCI 12.3 (534/4357) 12.8 (556/4352) 0.48 Previous stroke 3.1 (133/4357) 3.1 (136/4352) 0.85 25.8 (1123/4357) 26.0 (1130/4352) 0.85 8.2 (356/4357) 8.8 (384/4352) 0.28 Insulin dependent Current smoker p value Procedure indication All (acute) myocardial infarctions ST elevation myocardial infarction Non–ST-elevation myocardial infarction 17.6 (767/4357) 17.1 (746/4352) 0.57 Unstable angina 18.3 (796/4357) 19.3 (842/4352) 0.21 Stable angina 49.5 (2156/4357) 48.3 (2101/4352) 0.27 Silent ischaemia 6.5 (282/4357) 6.4 (279/4352) 0.93 Left ventricular ejection fraction (%) (n = 4489) 58.8±12.6 58.3±12.6 0.17 Serum creatinine (µmol/L) (n = 8152) 87.6±31.5 88.3±38.4 0.37 Complex patients* 58.0 (2526/4357) 58.1 (2528/4352) 0.93 Data given as percentage (n/N) or mean±standard deviation. * Defined as placement of a stent in a patient with at least one of the following clinical or lesion characteristics: renal insufficiency (creatinine level: ≥140 μmol/L [1·6 mg/dL]), ejection fraction: <30%, acute myocardial infarction ≤72 hours, >1 lesion per vessel, >2 vessels with stents, lesion length >27 mm, bifurcation lesion, lesion in bypass graft, in-stent restenosis, unprotected left main artery, lesion with thrombus, or total occlusion.7 CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention. Supplementary Table 2B. Baseline Lesion and Procedure Characteristics Variable E-ZES C-SES (n = 4357 patients) (n = 4352 patients) (n = 6151 lesions) (n = 6139 lesions) p value Lesion characteristics Vessel location (by lesion) 0.13 Left anterior descending 47.1 (2900/6151) 46.0 (2827/6139) Left circumflex 23.0 (1417/6151) 22.5 (1380/6139) Right coronary artery 28.9 (1776/6151) 30.1 (1850/6139) Left main 0.7 (43/6151) 1.0 (61/6139) Bypass graft 0.2 (15/6151) 0.3 (21/6139) Restenosis after previous PTCA 0.3 (18/6150) 0.4 (26/6139) 0.23 In-stent restenosis 1.0 (63/6150) 1.1 (65/6139) 0.86 Chronic total occlusion* 2.9 (177/6149) 2.7 (168/6139) 0.66 Bifurcation 16.9 (1038/6149) 15.9 (977/6139) 0.15 Moderate/severe calcification (vs none or mild) 27.8 (956/6149) 30.2 (1851/6139) 0.004 Tortuosity: moderate or severe (vs mild) 22.7 (1394/6146) 23.0 (1414/6139) 0.68 TIMI flow 0 or 1 15.5 (956/6149) 14.4 (887/6139) 0.09 Thrombus 7.6 (467/6149) 7.9 (486/6139) 0.52 ACC/AHA lesion class B2/C 53.7 (3300/6147) 55.7 (3421/6139) 0.025 Reference vessel diameter (mm) 3.0±0.5 (N=6141) 3.0±0.5 (N=6125) 0.030 Minimal luminal diameter (mm) 0.5±0.4 (N=6142) 0.5±0.4 (N=6127) 0.99 Diameter stenosis (%) 82.8±13.0 (N=6146) 82.7±12.8 (N=6129) 0.79 Lesion length (mm) 17.7±9.3 (N=6141) 17.7±9.1 (N=6125) 0.74 Number of vessels treated per patient 1.20±0.45 1.20±0.46 0.46 Number of lesions treated per patient 1.40±0.71 1.39±0.71 0.84 Number of stents per patient 1.63±0.99 1.59±0.96 0.054 Procedure characteristics Variable E-ZES C-SES p value (n = 4357 patients) (n = 4352 patients) (n = 6151 lesions) (n = 6139 lesions) Total stent length/patient (mm) 31.28±20.80 31.20±20.75 0.85 Number of stents per lesion 1.16±0.49 1.13±0.46 0.001 Lesions with pre-dilatation 67.5 (4152/6151) 69.4 (4262/6140) 0.023 Unfractionated heparin 92.1 (4011/4356) 92.0 (4003/4351) 0.91 Low-molecular-weight heparin 5.0 (216/4356) 5.4 (234/4351) 0.38 Direct thrombin inhibitor 4.2 (182/4356) 3.8 (167/4351) 0.44 Glycoprotein IIb/IIIa inhibitor 17.9 (780/4356) 18.4 (799/4351) 0.60 Residual stenosis (%) 1.9±9.2 2.2±10.1 0.097 TIMI 2/3 99.4 (6116/6151) 99.4 (6104/6139) 1.00 Lesion success† 99.6 (6032/6055) 99.4 (6006/6044) 0.055 Device success‡ 96.9 (5869/6055) 95.5 (5773/6044) <0.0001 Procedure success§ 97.0 (4145/4272) 96.9 (4135/4266) 0.75 Peri-procedure medication Post-procedure characteristics Data given as percentage (n/N) or mean±standard deviation * TIMI 0; no unstable angina; no myocardial infarction within 72 hours. † Attainment of <50% residual stenosis of the target lesion using any percutaneous method. ‡ Attainment of <50% residual stenosis of the target lesion using only the assigned device. § Attainment of <50% residual stenosis of all the target lesions and no in-hospital MACE. ACC/AHA, American College of Cardiology/American Heart Association; PTCA, percutaneous coronary angioplasty; TIMI, Thrombolysis In Myocardial Infarction. Supplementary Figures Supplmentary Figure 1. Usage of dual antiplatelet therapy at discharge, 6 months, and annually through 4 years. E-ZES=Endeavor zotarolimus-eluting stent. C-SES=Cypher sirolimus-eluting stent. Dual antiplatelet therapy defined as aspirin and a thienopyradine (clopidogrel, ticlopidine, or other). Supplementary Figure 2. Anticipated 3-year (A) and actual 4-year (B) rates of definite or probable stent thrombosis A. Anticipated 3-year rate E-ZES=Endeavor zotarolimus-eluting stent. C-SES=Cypher sirolimus-eluting stent. B. Actual 4-year rate E-ZES=Endeavor zotarolimus-eluting stent. C-SES=Cypher sirolimus-eluting stent.