THE DECOMPOSITION OF HYDROGEN PEROXIDE Introduction
advertisement

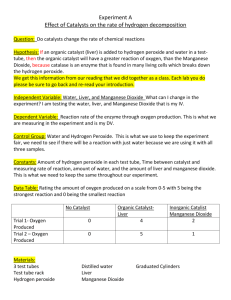
THE DECOMPOSITION OF HYDROGEN PEROXIDE Introduction Hydrogen peroxide (H2O2) readily decomposes to form water and oxygen gas in the presence of a manganese (IV) oxide (MnO2) catalyst: 2H2O2(aq) H2O(l) + O2(g) In this experiment you will measure the enthalpy change of this reaction and then compare the experimental value with both the theoretical value (determined from enthalpies of formation – HL students will learn about this in a couple of lessons time) and with the same value calculated from average bond enthalpies. Materials and Equipment Manganese (IV) oxide 20 vol hydrogen peroxide solution Standard laboratory equipment Preliminary Work 1. You will be using hydrogen peroxide solution with a concentration of ‘20 vol’. This means that one volume of hydrogen peroxide produces twenty volumes of oxygen gas. Use this information along with the reaction equation to determine the molar concentration of hydrogen peroxide. 2. Use average bond enthalpies to calculate roughly what you expect Hro to be (for the balanced equation: i.e. two moles of H2O2). 3. Use your answers to 1 and 2 to determine roughly how much you expect to see the temperature rise by if you use 20 vol H2O2. 4. Use your answer to 3 to help decide a suitable dilution of 20 vol H2O2 to use. Procedure Design and follow a suitable procedure to measure the energy change when 50 cm 3 of a suitable concentration of H2O2(aq) undergoes decomposition catalysed by 0.5g MnO2(s). If you use a similar calorimeter to that used in the ‘measuring an enthalpy change of reaction’ experiment, you can assume it has the same heat capacity, if not you will need to determine this too. Ensure you complete enough trials to gain reliable results. Analysis a) Determine the heat change observed in your experiment and thus the overall enthalpy change of reaction. b) How does your result compare with the theoretical value calculated in question 2? What are the reasons for any difference? c) How does the theoretical value of Hro compare with the one calculated from average bond enthalpies? What are the reasons for any difference? d) Use the idea of bond enthalpies to calculate the energy change expected for the following reactions. In which one would you expect the theoretical and experimental values to be most different? a. C2H6(g) + O2(g) CO2(g) + H2O(l) b. C4H8(g) + H2(g) C4H10(g) c. 6CO2(g) + 6H2O(g) C6H12O6(g) + 6O2(g)