First Exam

First Exam, CHEN313-Materials, Fall 2013, (90 minutes, Closed book)
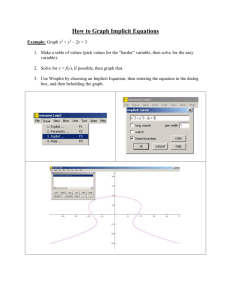
1. The simple model that approximates the interaction between a pair of neutral atoms is:
Mathematically determine r
0
and E
0
in terms of and
ANSWER: Just find the r = r o
that makes solving
That yields an imaginary number r o
=(-2) 1/6 σ
Then substitute it into E and get
E o
= -ε
2. Circled the configuration that corresponds to a transition metal
(a) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2
(b) 1s 2 2s 2 2p 6 3s 2 3p 6
(c) 1s 2 2s 2 2p 5
(d) 1s 2 2s 2 2p 6 3s 2
(e) 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2
(f) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1
ANSWER: For some chemists a transition metal is one with incomplete d orbitals; for others is any atom in the D-block; thus these two answers were given credit full credit:
(a), (e)
(e)
3. Calculate the ionic character of TiO
2
ANSWER: From the Table, the electronegativities are 1.5 and 3.5 for Ti and O, respectively, yielding
63.2%
4. Calculate the theoretical density in g/cm 3
ANSWER: Let’s do it from scratch now:
of NaCl
Na+ radius = 0.102 nm
Cl radius = 0.181 nm
Thus r
C
/r
A
ratio = 0.5635
Calculate the minimum ratio for coordination number = 8, do the geometry and get the ratio = 0.732
Calculate the minimum ration for coordination number = 6, do the geometry and get the ratio =0.414 (this was done in detail in class)
You can continue calculating minimum ratios for coordination numbers 4, and 3 but it is not needed.
Since NaCl should be coordination number 6, because
0.414 < 0.5635 < 0.732
Now you know the geometry, so you can construct a cell, for instance, the geometry of Figure 3.5 and from there find the density of 2.14 g/cm
5. Find out the cation to anion radius ratio for the coordination number 3
ANSWER: from cos30 =R
A
/(R
A
+R
C
) = sqrt(3)/2, yields R
C
/R
A
= 0.155
6. Determine the four indices for the directions shown in the following hexagonal unit cells :
ANSWER: [ 2 1] and [1 1 0]
7. Determine the Miller indices for the planes shown in the following cells
ANSWER: (3 4), (2 2 1), (0 0 0 2), (1 0 0)
8. What is the distance between the planes shown in the figure? Assume a perfect cubic lattice of 0.20 nm.
ANSWER: Plotting the front plane, we just need to find out the length of the red line. Certainly, there are several ways to solve this problem
In the internal rectangle, the diagonal length is sqrt(5)a/2 and the angle α is defined. Notice that the red line length is (a/2)cosα = (a/2)a/(sqrt(5)a/2 = a/sqrt(5) = 0.089 nm
Please let me know if you have any comments or questions