PROBLEM SET Thermodynamics Review Pack
advertisement
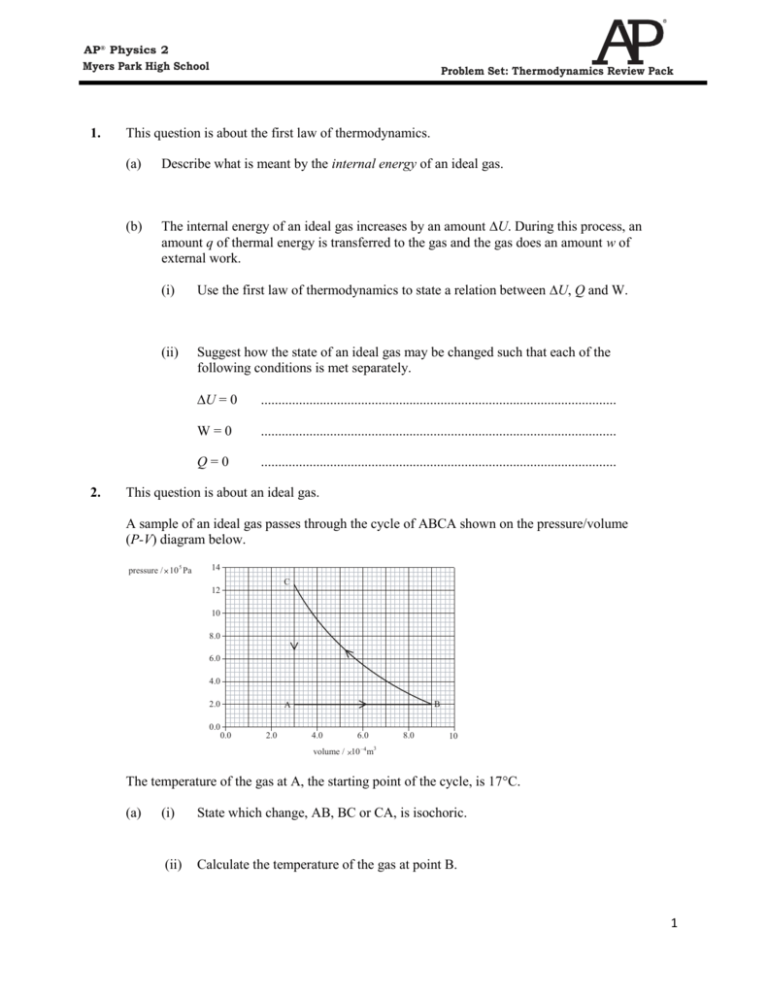
AP® Physics 2 Myers Park High School 1. 2. Problem Set: Thermodynamics Review Pack This question is about the first law of thermodynamics. (a) Describe what is meant by the internal energy of an ideal gas. (b) The internal energy of an ideal gas increases by an amount U. During this process, an amount q of thermal energy is transferred to the gas and the gas does an amount w of external work. (i) Use the first law of thermodynamics to state a relation between U, Q and W. (ii) Suggest how the state of an ideal gas may be changed such that each of the following conditions is met separately. U = 0 ....................................................................................................... W=0 ....................................................................................................... Q=0 ....................................................................................................... This question is about an ideal gas. A sample of an ideal gas passes through the cycle of ABCA shown on the pressure/volume (P-V) diagram below. pressure / 10 5 Pa 14 C 12 10 8.0 6.0 4.0 2.0 0.0 0.0 B A 2.0 4.0 6.0 8.0 10 volume / 10 –4 m3 The temperature of the gas at A, the starting point of the cycle, is 17C. (a) (i) State which change, AB, BC or CA, is isochoric. (ii) Calculate the temperature of the gas at point B. 1 AP® Physics 2 Myers Park High School (iii) 3. Problem Set: Thermodynamics Review Pack Calculate the temperature of the gas at point C. (b) During the change AB, 300 J of thermal energy is supplied to the gas. Determine the change in internal energy of the gas. (c) During the change BC, 250 J of thermal energy is transferred. The area ABC on the pressure/volume diagram represents 120 J of energy. Calculate the thermal energy transfer during the stage CA. Explain your working. This question is about entropy changes. (a) State what is meant by an increase in entropy of a system. (b) State, in terms of entropy, the second law of thermodynamics. (c) When a chicken develops inside an egg, the entropy of the egg and its contents decreases. Explain how this observation is consistent with the second law of thermodynamics. 2 AP® Physics 2 Myers Park High School 4. Problem Set: Thermodynamics Review Pack Expansion of a gas An ideal gas at an initial pressure of 4.0 105 Pa is expanded isothermally from a volume of 3.0 m3 to a volume of 5.0 m3. (a) Calculate the final pressure of the gas. (b) On the axes below draw a sketch graph to show the variation with volume V of the pressure p during this expansion. p / 105 Pa 6 4 2 0 0 (c) 2 4 6 / m3 Use the sketch graph in (b) to (i) estimate the work done by the gas during this process; (ii) explain why less work would be done if the gas were to expand adiabatically from the same initial state to the same final volume. 3 AP® Physics 2 Myers Park High School 5. Problem Set: Thermodynamics Review Pack This question is about thermodynamic processes. (a) Distinguish between an isothermal process and an adiabatic process as applied to an ideal gas. An ideal gas is held in a container by a moveable piston and thermal energy is supplied to the gas such that it expands at a constant pressure of 1.2 × 105 Pa. thermal energy piston The initial volume of the container is 0.050 m3 and after expansion the volume is 0.10 m3. The total energy supplied to the gas during the process is 8.0 × 103 J. (b) (i) State whether this process is either isothermal or adiabatic or neither. (ii) Determine the work done by the gas. (iii) Hence calculate the change in internal energy of the gas. 4 AP® Physics 2 Myers Park High School 6. Problem Set: Thermodynamics Review Pack This question is about p–V diagrams. The graph below shows the variation with volume of the pressure of a fixed mass of gas when it is compressed adiabatically and also when the same sample of gas is compressed isothermally. 7.0 C 6.0 5.0 B pressure / x 105 Pa 4.0 3.0 2.0 1.0 A 2.0 3.0 4.0 –3 3 volume / x 10 m 5.0 6.0 (a) State and explain which line AB or AC represents the isothermal compression. (b) On the graph, shade the area that represents the difference in work done in the adiabatic change and in the isothermal change. (c) Explain how the difference in work done, as identified in (b). (d) Use the first law of thermodynamics to explain the change in temperature during the adiabatic compression. 5 AP® Physics 2 Myers Park High School 7. Problem Set: Thermodynamics Review Pack The diagram below shows the relation between the pressure and the volume of the air in the engine for one cycle of operation of the engine. pressure B C D A volume (i) State the name given to the type of process represented by DA. (ii) During one cycle of the engine, the gas absorbs Q1 units of thermal energy and Q2 units of thermal energy are transferred from the gas. On the diagram above, draw labelled arrows to show these energy transfers. (iii) The efficiency of the engine is 60. Using your answer to question (i), calculate the values of Q1 and Q2. 8. A sample of an ideal gas is contained in a cylinder fitted with a piston, as shown below. Piston Ideal gas Cylinder (a) (i) Explain, in terms of molecules, what is meant by the internal energy of the gas. (ii) The piston is suddenly moved inwards, decreasing the volume of the gas. By considering the speeds of molecules, suggest why the temperature of the gas changes. 6 AP® Physics 2 Myers Park High School (iii) Problem Set: Thermodynamics Review Pack The gas now expands at constant pressure p so that the volume increases by an amount ΔV. Derive an expression for the work done by the gas. An engine operates by using an isolated mass of an ideal gas. The gas is compressed adiabatically and then it is heated at constant volume. The gas gains 310 J of energy during the heating process. The gas then expands adiabatically. Finally, the gas is cooled so that it returns to its original state. During the cooling process, 100 J of energy is extracted. The cycle is shown below. C pressure / Pa 310 J 6.1×10 6 B D 100 J 1.0×10 5 A 300 K 0.32×10–4 6.0×10–4 volume / m3 (b) (i) Mark, on the diagram, arrows to show the direction of operation of the stages of the cycle. (ii) Using data for point A, calculate the number of moles of gas. (iv) Determine the temperature of the gas at point B in the cycle. 7 AP® Physics 2 Myers Park High School (v) Problem Set: Thermodynamics Review Pack State what is represented by the area ABCD on the diagram and give the value of this quantity. (vi) Calculate the efficiency of the engine. 9. This question is about thermodynamic processes. (a) On the diagram below, draw arrows to show the energy transfers associated with a heat pump. hot resevoir cold resevoir 8 AP® Physics 2 Myers Park High School (b) Problem Set: Thermodynamics Review Pack The diagram below, shows the relation between the pressure P and the volume V of the working substance of the heat pump for one cycle of its operation. P B A C D V (i) The working substance at point C of the cycle is in the liquid phase. State the reason why both the changes from C D and A B are isothermal isobaric changes. (ii) State during which process of the cycle energy is absorbed from the cold reservoir and during which process energy is transferred to the hot reservoir. (iii) State how the value of the work done during one cycle may be determined from the PV diagram. 9 AP® Physics 2 Myers Park High School Problem Set: Thermodynamics Review Pack Thermal Physics Worksheet Multiple Choice 10 AP® Physics 2 Myers Park High School Problem Set: Thermodynamics Review Pack 10. The equation of state for an ideal gas, pV = nRT, describes the behaviour of real gases A. only at low pressures and large volumes. B. only at high temperatures. C. only at large volumes and large pressures. D. at all pressures and volumes. 11. The second law of thermodynamics states that the entropy of the universe is A. increasing. B. decreasing. C. zero. D. constant but not zero. 12. When a gas in a thermally insulated cylinder is suddenly compressed, the change of state is A. adiabatic. B. isothermal. C. isobaric. D. isochoric. 11 AP® Physics 2 Myers Park High School Problem Set: Thermodynamics Review Pack 13. The temperature of an ideal gas is reduced. Which one of the following statements is true? A. The molecules collide with the walls of the container less frequently. B. The molecules collide with each other more frequently. C. The time of contact between the molecules and the wall is reduced. D. The time of contact between molecules is increased. 14. A gas is contained in a cylinder fitted with a piston as shown below. piston gas When the gas is compressed rapidly by the piston its temperature rises because the molecules of the gas A. are squeezed closer together. B. collide with each other more frequently. C. collide with the walls of the container more frequently. D. gain energy from the moving piston. 15. The graph below shows the variation with volume of the pressure of a system. 5 Pressure / 10 Pa 5 Q 4 3 2 1 P R 0 0 1 2 3 4 5 6 Volume / m3 The work done in compressing the gas from R to P is A. 5 5.0 × 10 J. B. 5 4.5 × 10 J. C. 5 3.0 × 10 J. D. 0. 16. The graph below shows the variation with volume V of the pressure p of a gas during one cycle of an engine. 12 AP® Physics 2 Myers Park High School Problem Set: Thermodynamics Review Pack p R Q S P V During which operations, PQ, QR, RS and SP does the gas do external work? A. PQ only B. RS only C. QR and RS only D. PQ and RS only 17. A gas expands rapidly. The process is approximately A. isobaric. B. isothermal. C. adiabatic. D. isovolumetric. 18. A sample of an ideal gas is held in an insulated container and it undergoes an adiabatic change. The graph below shows the change in pressure p with change in volume V as the gas changes from X to Y. p Y X V Which of the following describes correctly the work done and the change in the internal energy of the gas? Work done Internal energy A. on the gas increases B. on the gas decreases C. by the gas decreases D. by the gas increases 13 AP® Physics 2 Myers Park High School Problem Set: Thermodynamics Review Pack 19. The diagram shows the pressure/volume (p/V) diagram for one cycle PQRS of an engine. p Q R S P V In which sections of the cycle is work done on the engine? A. SP only B. PQ only C. SP and PQ only D. RS and SP only 14








