SUPPLEMENTAL MATERIALS Extraction optimization and isolation
advertisement

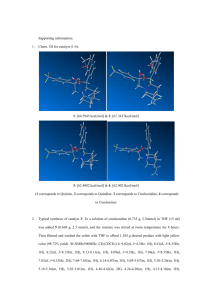
SUPPLEMENTAL MATERIALS Extraction optimization and isolation of triterpenoids from Ganoderma lucidum and their effect on human carcinoma cell growth Weimei Ruan1, Lim Hiang He Andrew1, Lau Gek Huang1 and David G. Popovich2* 1 2 Department of Chemistry, National University of Singapore, Singapore Institute of Food, Nutrition and Human Health, College of Health, Massey University, Palmerston North, New Zealand *Corresponding author: Tel: +64 6 350 5904 Fax: +64 6 350 5657 Email address: D.G.Popovich@massey.ac.nz ABSTRACT The response surface methodology (RSM) was used to optimize the extraction conditions of Ganoderma lucidum based on a Box–Behnken design (BBD). A quadratic model sufficiently simulated the response of ganoderic acid H with a determination coefficient (R2) of 0.98. The optimal condition for extracting triterpenoids was determined to be 100.00% ethanol at 60.22 °C for 6.00 h, under which the yield of the reference triterpenoid ganoderic acid H increased from 0.88 to 2.09 mg/g powder. Following extraction, triterpenoid enriched fraction was further isolated into 23 fractions and 7 fractions were identified as ganoderic acid A, B, D, G, H and I and ganoderenic acid D. Of the 7 triterpenoids, ganoderenic acid D was most cytotoxic with LC50s of 0.14±0.01, 0.18±0.02 and 0.26±0.03 mg/mL in Hep G2, Hela and Caco-2 cells respectively. While ganoderic acid A, G and H were relatively non-cytotoxic. The variation of inhibitory effects for these triterpenoids was likely related to their chemical structures. 1 The molecular weight and structural details of triterpenoids isolated from Ganoderma lucidum triterpenoid enriched extract are: Ganoderic acid I (3.63 mg from 180 mg fraction 2) ESI-MS m/z: 531 [M-H]-, 1063[2M-H]-,513,469,451, 303, 265. 1H NMR δCDCl3 : 3.21 (1H, dd, J=6.0, 11.2Hz, 3-H), 0.87 (1H, dd, J=1.9, 11.0Hz, 5-H), 4.79 (1H, t, J=8.4Hz, 7-H), 1.14 (3H, s, 18-H), 1.22 (3H, s, 19-H), 1.39 (3H, s, 21-H), 0.85 (3H, s, 31-H), 1.34 (3H, s, 32-H). 13 C NMR δCDCl3 : 34.9, 27.7, 78.3, 38.8, 49.2, 26.6, 66.9, 156.7, 142.5, 38.7, 198.0, 50.6, 45.6, 59.7, 217.7, 36.1, 49.1, 19.0, 18.4, 73.0, 26.6, 52.6, 210.4, 47.5, 34.2, 175.2, 16.9, 28.2, 15.4, 24.8. Ganoderic acid G (4.41 mg from 180 mg fraction 2) ESI-MS m/z: 531 [M-H]-, 1063[2M-H]-,513,469, 319, 303, 265. 1H NMR δCDCl3 : 3.21 (1H, dd, J=7.6, 8.8Hz, 3-H), 4.77 (1H, t, J=8.4Hz, 7-H), 4.36 (1H, s, 12-H), 0.79 (3H, s, 18-H), 1.30 (3H, s, 19-H), 1.13 (3H, d, J=6.4Hz, 21-H), 1.21 (3H, d, J=8.0Hz, 26-H), 1.03 (3H, s, 30-H), 0.87 (3H, s, 31-H), 1.44 (3H, s, 32-H). 13 C NMR δCDCl3 : 34.3, 27.5, 78.3, 38.6, 49.1, 26.7, 66.2, 157.3, 141.9, 38.3, 199.3, 77.8, 51.9, 60.3, 216.8, 38.2, 45.7, 11.9, 18.8, 28.7, 21.3, 48.3, 8.2, 46.2, 34.5, 176.1, 16.9, 28.1, 15.3, 23.0. Ganoderic acid B (3.42 mg from 180 mg fraction 2) ESI-MS m/z: 515 [M-H]-, 1031[2M-H]-, 497, 453 , 303, 287, 249. 1H NMR δCDCl3 : 3.21 (1H, dd, J=6.0, 11.2Hz, 3-H), 0.87 (1H, dd, J=3.6, 8.3Hz,5-H), 4.79(1H, t, J=8.4Hz, 7-H), 1.00 (3H, s, 18-H), 1.21 (3H, s, 19-H), 0.98 (3H, d, J=6.0Hz, 21-H), 1.24 (3H, d, J=6.4Hz, 26-H), 1.03 (3H, s, 31-H), 1.34 (3H, s, 32-H). 13 C NMR δCDCl3 : 34.3, 28.1, 78.3, 38.8, 45.3, 27.6, 66.8, 156.8, 142.7, 45.5, 197.8, 50.3, 38.6, 59.4, 207.6, 40.9, 49, 16.9, 17.4, 31.9, 19.6, 49.1, 217.5, 46.5, 34.8, 26.6, 179.5, 15.4, 24.4, 18.4. Ganoderic acid A (5.70 mg from 180 mg fraction 2) ESI-MS m/z: 515 [M-H]-, 1031[2M-H]-, 497, 435, 405, 301, 285. 1H NMR δCDCl3 : 4.62(1H, t, J=8.0Hz, 7-H), 4.79 (1H, t, J=8.0Hz,15-H), 0.98 (3H, s, 18-H), 1.25 (3H, s, 19-H), 0.88 (3H, d, J=6.0Hz, 21-H), 1.24 (3H, d, J=7.3Hz, 26-H), 1.1 (3H, s, 30-H), 1.12 (3H, s, 31-H), 1.28 (3H, s, 32-H). 13 C NMR δCDCl3 : 35.6, 34.3, 208.5, 46.8, 48.9, 29.2, 68.9, 159.0, 140.6, 46.6, 200.2, 51.7, 38.0, 54.0, 72.5, 36.3, 47.9, 17.3, 19.5, 32.6, 19.3, 49.5, 216.9, 46.4, 34.5, 27.3, 179.8, 16.9, 20.7, 19.7. Ganoderic acid H (4.12 mg mg from 180 mg fraction 2) ESI-MS m/z: 571 [M-H]-, 1143[2M-H]-, 553, 511,467, 437, 319, 303, 301. 1H NMR δCDCl3 : 3.25 (1H, dd, J=7.6, 8.8Hz, 3-H), 5.63 (1H, s, 12-H), 0.82 (3H, s, 18-H), 1.33 (3H, s, 19-H), 0.99 (3H, d, J=6.4Hz, 21-H), 1.21 (3H, d, J=8.0Hz, 26-H), 1.03 (3H, s, 30-H), 0.88 (3H, s, 31-H), 1.73 (3H, s, 32-H). 13 C NMR δCDCl3 : 33.2, 2 27.3, 77.2, 40.4, 51.4, 36.6, 198.8, 151.7, 145.7, 39.1, 193.9, 79.2, 47.9, 58.4, 205.7, 37.9, 44.7, 12.2, 17.9, 29.4, 21.6, 48.3, 207.4, 46.4, 34.3, 176, 16.9, 27.9, 15.5, 21.3 Ganoderenic acid D (2.49 mg mg from 180 mg fraction 2) ESI-MS m/z: 511 [M-H]-, 1023[2M-H]-, 493, 449, 301, 285, 247, 149. 1H NMR δCDCl3 : 4.87 (1H, dd, J=7.6, 9.2Hz,7-H), 0.89 (3H, s, 18-H), 1.24 (3H, s, 19-H), 2.18 (3H, s, 21-H), 6.05 (1H, s, 22-H), 1.23 (3H, d, J=5.6Hz, 26-H), 1.13 (3H, s, 30-H), 1.11 (3H, s, 31-H), 1.40 (3H, s, 32-H). 13 C NMR δCDCl3 : 35.6, 34.3, 216.5, 46.7, 48.8, 27.6, 66.3, 157.4, 141.4, 38.2, 197.8, 48.9, 45.9, 58.6, 216.4, 37.8, 49.7, 19.0, 18.1, 153.8, 21.0, 124.6, 196.1, 47.5, 34.6, 180.6, 16.9, 27.0, 20.8, 24.7. Ganoderic acid D (2.71 mg from 180 mg fraction 2) ESI-MS m/z: 513 [M-H]-, 1027[2M-H]-, 495, 451, 301, 285, 247, 149. 1H NMR δCDCl3 : 4.80 (1H, t, J=8.8Hz, 7-H), 0.99 (3H, s, 18-H), 1.20 (3H, s, 19-H), 0.94 (3H, d, J=7.6Hz, 21-H), 1.21(3H, d, J=7.6Hz, 26-H), 1.06 (3H, s, 30-H), 1.03 (3H, s, 31-H), 1.30 (3H, s, 32-H). 13 C NMR δCDCl3 : 35.6, 34.3, 217.6, 46.8, 48.9, 27.6, 66.3, 157.8, 141.2, 38.2, 197.6, 50.1, 45.0, 59.3, 216.7, 41.0, 45.6, 17.6, 18.1, 32.0, 19.6, 48.9, 207.6, 46.6, 34.3, 179.4, 16.9, 27.0, 20.8, 24.7. 3 Table S1. Regression coefficients of predicted quadratic polynomial models for the yields of total extract and ganoderic acid H. Source X1 Sum of Squares total ganoderic extract acid H 7.61 0.037 df 1 Mean Square total ganoderic extract acid H 7.61 0.037 F Value total ganoderic extract acid H 40.20 8.33 p-value (Prob > F) total ganoderic extract acid H 0.0014 0.034 X2 1.21 0.39 1 1.21 0.39 6.42 88.62 0.052 0.0002 X3 1.17 0.024 1 1.17 0.024 6.17 5.43 0.056 0.067 X1 X2 6.53 0.13 1 6.53 0.13 34.52 29.54 0.0020 0.0029 X1 X3 0.37 0.039 1 0.37 0.039 1.93 8.81 0.22 0.031 X2 X3 10.08 0.22 1 10.08 0.22 53.28 49.42 0.0008 0.0009 X1 2 14.76 0.15 1 14.76 0.15 77.99 33.05 0.0003 0.0022 X2 2 0.071 0.049 1 0.071 0.049 0.37 11.20 0.57 0.020 X3 2 13.49 0.20 1 13.49 0.20 71.28 46.72 0.0004 0.0010 Model Pure Error 57.64 1.26 9 6.40 0.14 33.85 31.92 0.0006 0.0007 0.41 0.00071 2 0.20 0.00036 Total 58.59 1.28 14 X1 refers to extraction time; X2 refers to the ethanol ratio; X3 refers to the extraction temperature. 4 Table S2. ANOVA for the fitted quadratic polynomial model of extraction of total extract and ganoderic acid H. Total extract Ganoderic acid H Mean 8.79 1.40 Std. Dev. 0.44 0.066 R-Squared 0.98 0.98 Adj R-Squared 0.95 0.95 C.V. % 4.95 4.73 5 Figure S1. (a) Representative HPLC chromatogram of extraction of Ganoderma lucidum under Box–Behnken factorial design (BBD) conditions. Ganoderic acid H was calibrated using an external standard. (b) HPLC profile of triterpenoid enriched fraction (fraction 2) by semi-preparative HPLC isolation. Peak numbers refer to the collected fractions: 8, ganoderic acid I; 12, ganoderic acid G; 14, ganoderic acid B; 17, ganoderic acid A; 18, ganoderic acid H; 20, ganoderenic acid D; 21, ganoderic acid D. (c) Chemical structures of identified triterpenoids from Ganoderma lucidum.:”-“ refers to no attachment in this position. 6 7 Figure S2. Dose-response relationship of ganoderic acid A, B, D, G, H and I and ganoderenic acid D in (a) HeLa cells, (b) Caco-2 cells and (c) Hep G2 cells after 72 h treatment measured by MTT assay. Values are expressed as mean ± SD. 8