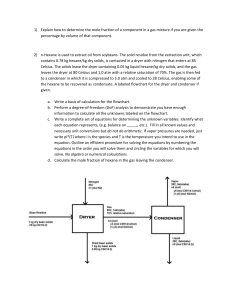
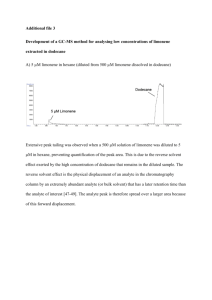
GC-MS and GC-FID

Gas Chromatography Flame Ionization Detector and Gas Chromatography Mass
Spectrometry
Introduction: GC with the flame ionization detector is generally used for volatile organic samples. The
GC-MS is a good tool for analysis of organic compounds. We will be running known samples of hydrocarbons on the GC-MS in order to determine their peaks (retention times). After we run a few known samples, we will then run our unknown sample mixture of hydrocarbons and attempt to determine the composition and relative concentrations based on the retention times and chromatograms of our known standards.
Purpose: We will be running known samples of hydrocarbons on the GC-FID in order to determine their retention times. After running a few known samples, we will then mix a few hydrocarbons and attempt to determine the resolution of the mixture by varying the concentration of the components in our mixture. After we get our resolution to a good point, we will then take an unknown sample that’s been prepared for us and run it on the column in an attempt to identify the components of the mixture. GC-
MS is a very common instrument that is used mostly to run organic samples. The mass-spectrometry aspect of the GC allows us to retrieve a mass to charge ratio of our different compounds which then allows us to identify them. The polarity of each compound is what determines the retention time of the analyte. The more polar the compound, the faster it comes off the column because we are using a nonpolar solvent to push the analyte onto the column.
Procedure:
GC-MS
1.
Check that the levels on the Helium tank are at least 80 psi
2.
Open the software by clicking on the first icon to the left at the very top, click on acquisition, go to file and activate the Instrumental 2012 GCMS method
3.
Click on the method bar and select view/edit method (at the very top)
4.
Under GC control, be sure that all split states are on
5.
Under column oven you can change the delay to get rid of the peak for hydrocarbon.
6.
If it is not ready, go to the method bar and reactivate it. If it continues to not say ready, then click on acquisition and reset.
7.
Wait for the software to say that it is ready and for the green “ready” button on the GC-
MS instrument turn on
8.
To prepare the sample, measure 15 mL of hexane into a 30 mL beaker and pipette 10-15 drops of analyte into beaker of hexane (allows for better chromatograms)
9.
Clean the syringe with acetone by filling it multiple times with acetone (to ensure the syringe is clean)
10.
Fill the syringe with one microliter of the sample and one micro liter of air
11.
Inject the sample in the middle of the silver circle on the top of the instrument. Be sure to push the needle all the way in (about ten minutes per sample)
12.
If the program doesn’t begin on its own, click start acquisition
13.
When the spectrum is complete, click on the MS Data review button (3 rd on the right)
Right click on the main peak and select Library Search Spectrum.
14.
Save the file and print the spectra (right click to print spectrum)
15.
Determine which peaks go with which elements in the sample.
16.
Close all windows when done but leave the tool panel (at the top of the screen) open.
GC-FID
17.
Slowly turn on the valves in the gas room (vertical is on), then turn the nozzles on the helium, compressed air, and hydrogen tanks. The pressure should be at 80 psi or higher. Helium should always be on and never turned off.
18.
Once gasses have been checked and turned on, slowly turn on the valves behind the instrument on for helium, compressed air, and hydrogen.
19.
Open software by clicking the first button (top left) on the horizontal menu bar. View/edit method and turn on injector, column oven, detector, and electronics. Never change front valve oven to yes under sample delivery. Under output port, A stays on. Choose existing method and
Instrumental 2012 GCMS (File > activate method)
20.
Lift the top of the instrument up to check the flame. Place metal spatula end over the small metal gap. If the metal fogs up, the flame is lit.
21.
If the flame is not lit, try re-activating the method by clicking on the method bar and selecting reactivate method.
22.
To make the samples, measure out 15 mL of hexane in a 30 mL beaker and dissolve 10-15 drops of sample into the beaker with hexane
23.
Fill your syringe with 1 microliter of sample and then pull in 1 microliter of air
24.
Wait for the computer to say it’s ready.
25.
Inject your sample into the middle of the silver circle on top of the instrument. Push all the way down then inject the sample and remove syringe.
26.
When sample is finished running, press the fourth button from the left (review/process MS data). To determine the area for various peaks, click on the fourth icon from the left above the graph and click integrate area. Drag the arrow along the bottom of the peak.
27.
Turn off air and hydrogen nozzles behind instrument. Do not turn off the helium
Data: The spectrum we obtained for the GC-MS are linked below, the spectrum from the GC-FID are in my notebook.
Hexane
Octane
Decane
Unknown Sample
Sample
Hexane
Octane
Decane
Unknown
Peak 1
Peak 2
Peak 3
Peak 4
Peak 5
Retention Time (min)
1.824
2.351
3.534
Peaks Retention Time
(min)
1.831
2.284
2.861
3.451
4.521
Hydrocarbon
Hexane
Octane
Nonane
Decane
Benzene
Conclusions: Overall, the experiment was a success. The GC-MS and the GC-FID are both relatively user friendly and easy to use. With the GC-MS unknown mixture, we were able to determine 3 components from our internal standards. The two peaks that were not matched with an internal standard were determined by some of other peaks we identified when we were doing our real world project. The mass spectrometry portion of the GC is very helpful in determining the unknown compounds because it gives you a molecular weight. The mixture contained hexane, octane, decane, benzene and nonane. The benzene and nonane peaks were the ones we had to rely on the mass spec to help us identify the peaks.
This experiment would have been more successful if we had run more standards.