Quantum Theory Introduction
advertisement

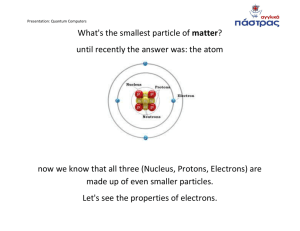
Quantum Theory and Quantum numbers Electrons behave like waves. They give off wavelengths of light dependent on the energy level to which they move. c= λѵ this equation means… E=hѵ this equation means… Symbols, units are ? Must use proper format for problem solving to earn full credit. P. 140 and 143 problems in text for hw Scientific notation must be used when doing these equations. Use on calculator or do long hand. When dividing exponents, subtract When multiplying exponents, add λ= h/mv wavelength of energy emitted by electrons dependent on Planck’s /momentum of electron; its mass x its velocity λ= h/mv Units for each variable? Bohr Model; using hydrogen- he stated… De Broglie told us what? Shrodinger established sublevels called What are they? How many electrons can the s, p, d, f, suborbitals contain? Using packet information on wires called quantum numbers tutorial, what are the principles of Aufbau and Pauli Exclusion and Hund Rule? Explain each and give an example using a particular element. What is the order for the first six sublevels and the numbers of e- they may contain. The order is the order in which the e- do what? Record the e- config for the following elements and diagram the orbital boxes using the rules of Hund, Aufbau, and Pauli Calcium Magnesium Fluorine Silicon Sodium Oxygen Helium Argon What makes the noble gases different from all other elemets in terms of the e- config? Why does each period end in a noble gas? Read in packet about Lewis dot diagrams. What do they represent? What is a valence electron(s)?