Schedule - Lewiston Independent School District #1
advertisement
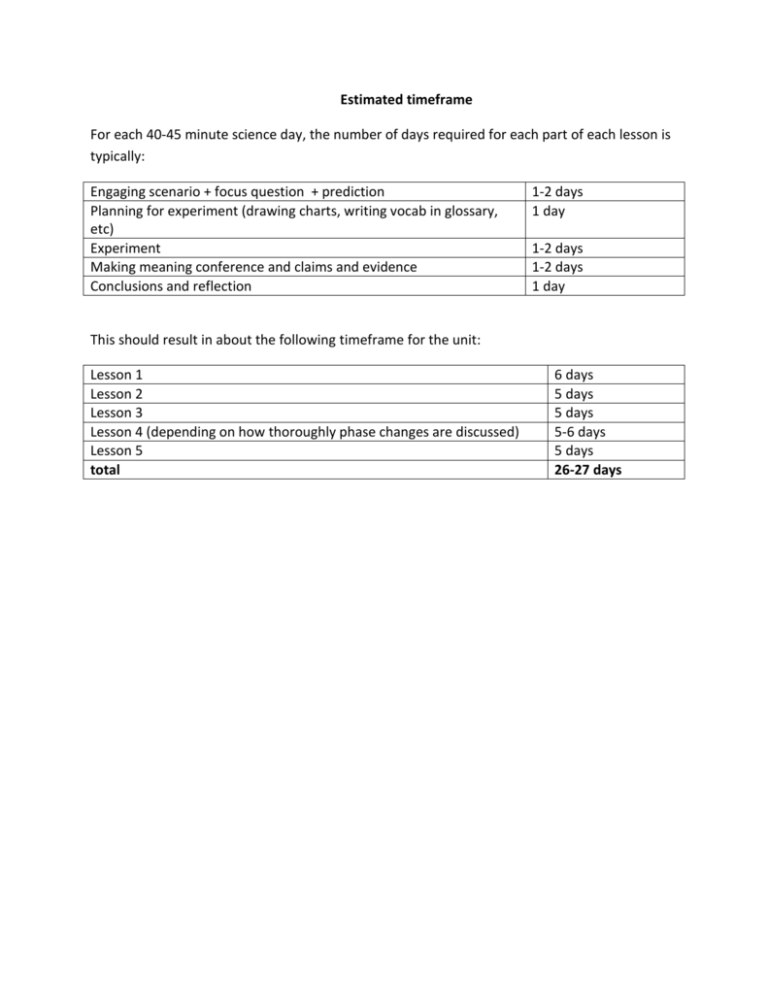
Estimated timeframe For each 40-45 minute science day, the number of days required for each part of each lesson is typically: Engaging scenario + focus question + prediction Planning for experiment (drawing charts, writing vocab in glossary, etc) Experiment Making meaning conference and claims and evidence Conclusions and reflection 1-2 days 1 day 1-2 days 1-2 days 1 day This should result in about the following timeframe for the unit: Lesson 1 Lesson 2 Lesson 3 Lesson 4 (depending on how thoroughly phase changes are discussed) Lesson 5 total 6 days 5 days 5 days 5-6 days 5 days 26-27 days Introduction to chemistry and the Big Idea of this unit Thank you so much for teaching the Mixtures and Solutions Unit. If you have any corrections, feedback, comments, or questions about the unit, please do not hesitate to contact me at rajameton@lcsc.edu. In this note, I want to offer some background for the BIG IDEA and also the matter chart that appears throughout the unit, which is duplicated on the next page. There is also a timeline for the unit and a summary of the vocabulary for your wordwall. So, here is the background for the BIG IDEA: At the heart of chemistry is the desire to explain how matter behaves. Matter is the stuff around us with mass, and includes flowers, people, furniture, cars, food, everthing. When chemists ask questions about matter, they must be very specific about the type of matter that they are talking about. The questions that a chemist asks about flowers are very different than the questions the chemist asks about a car! Here are some examples of the sorts of questions that a chemist might want to find out about different types of matter: What causes flowers to have their specfic colors and fragrances? How can we make plastics that are flexible yet strong? Why are diamonds so hard? Will a new pesticide hurt fish if it is released into the wate?r Can a drug be made that better treats MRSA? Or cancer? Or the common cold? Or heart disease? How can we make ice colder so that we can make great ice cream? The lists of questions are endless! Try taking an object, like your pen, and think of all the questions that you could ask about its physical nature: Why is the plastic rigid? Can you melt the plastic? What makes the ink black? What makes the ink impossible to erase? How is the plastic given its shape? And the list goes on and on. At the heart of how chemists explain all of these different properties is the notion of the atom. An atom is a small unit of matter that comes in 115 different types called elements. Most of the materials around us are composed of just a few elements: carbon, nitrogen, hydrogen, oxygen and a little phosphorus, sulfur, sodium and magnesium. So how then, do we get the incredible varied types of matter around us like stuffed animals, DNA, plastic bags, crayons, chocolate chip cookies and water? It is because atoms combine in different ways to give compounds. Compounds, also called molecules, are specific arrangements of atoms. For example, a water molecule is composed of two hydrogens and an oxygen. Compounds can be as small as two atoms, up to many thousands of atoms in proteins and plastics. The matter that we can see contains many, many molecules. Occasionally, the matter that we see is composed of one type of molecule, and is called a pure compound. Most of the time, matter is a combination of many different types of molecules, and these are mixtures. You may notice that many of the terms that I used above (matter, elements, pure compounds and mixtures) are shown in the figure below. I meant for that to happen! The figure below shows how chemists boradly organize matter: we divide anything that we encounter into mixtures or pure substances and then further subdivide it as shown below. Much in the way that a biologist organizes living organisms into kingdoms and phyla, doing this categorization helps chemists organize and describe the enormous amount of matter around us and begin to describe some of its differences and similarities. Pure substances (LE2 and LE3) Solutions (LE2) Mixtures (LE1) Over the course of this unit, you will introduce your students to the terms in the chart through specific examples of materials in their everyday life such as salt and water. Furthermore, you and your students will explore similarities within these categories. All mixtures, for example, can be separated. You will also investigate how different types of matter within a single category can differ, leading to the BIG IDEA: “Elements and their combinations account for all the varied types of matter around us.” Unit Vocabulary (in order of appearance in each lesson) Lesson 1 2 Term Mixture Solid Liquid Property Pure Substance A solution Solvent Solute Evaporation Crystal 3 4 Solid (repeated from 1) Atom Element Freeze Freezing point Concentration Saturated Solubility Phase change 5 Melt Vaporize Condense No new vocab Definition a combination of materials matter with definite shape and volume matter with a definite shape but indefinite volume a characteristic feature a single type of matter (an element or pure compound) a mixture of two or more different substances where one substance dissolves in another. The major component(s) of a solution The minor component(s) of a solution Change of state from a liquid to a gas A solid material with a specific and repeating pattern of atoms matter with definite shape and volume The smallest unit that makes up all matter A type of atom To change from a liquid to a solid (opposite of melting) the temperature at which a liquid freezes. the amount of one compound in a mixture. A solution with the maximum amount of a solute dissolved in it. Solubility: the amount of a solute in a saturated solution. When a solid, liquid or gas changes to a different state of matter. To change from a solid to a liquid. To change from a liquid to a gas. To change from a gas to a liquid.















