File
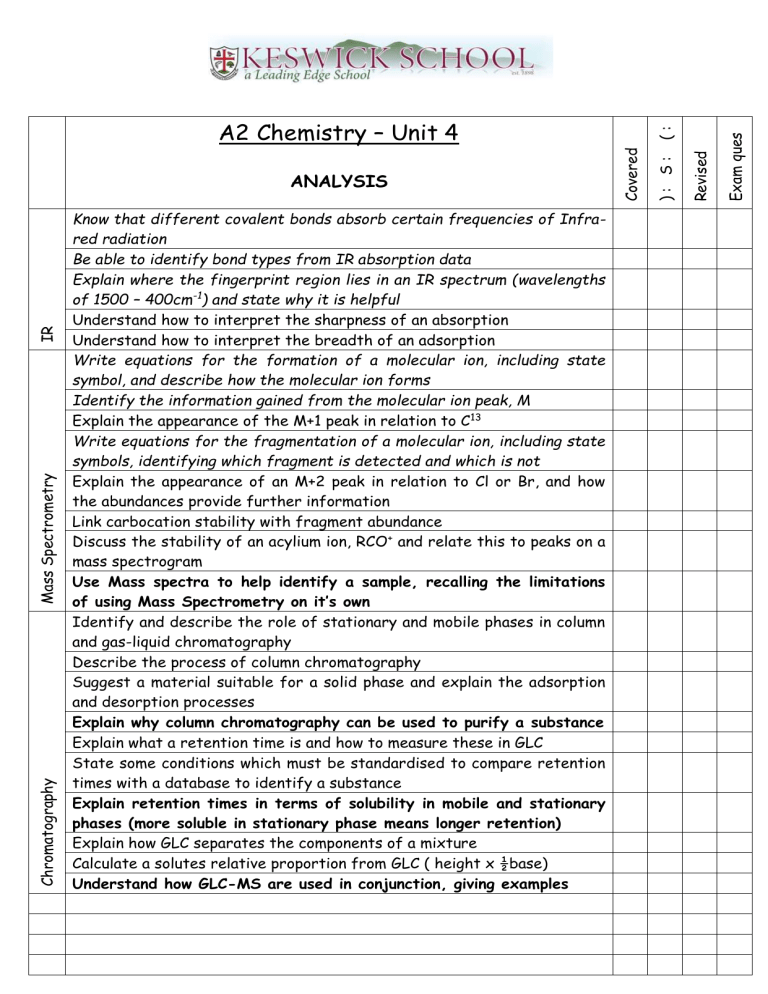
A2 Chemistry – Unit 4
ANALYSIS
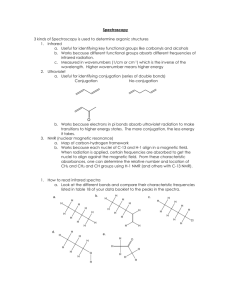
Know that different covalent bonds absorb certain frequencies of Infrared radiation
Be able to identify bond types from IR absorption data
Explain where the fingerprint region lies in an IR spectrum (wavelengths of 1500 – 400cm -1 ) and state why it is helpful
Understand how to interpret the sharpness of an absorption
Understand how to interpret the breadth of an adsorption
Write equations for the formation of a molecular ion, including state symbol, and describe how the molecular ion forms
Identify the information gained from the molecular ion peak, M
Explain the appearance of the M+1 peak in relation to C 13
Write equations for the fragmentation of a molecular ion, including state symbols, identifying which fragment is detected and which is not
Explain the appearance of an M+2 peak in relation to Cl or Br, and how the abundances provide further information
Link carbocation stability with fragment abundance
Discuss the stability of an acylium ion, RCO + and relate this to peaks on a mass spectrogram
Use Mass spectra to help identify a sample, recalling the limitations of using Mass Spectrometry on it’s own
Identify and describe the role of stationary and mobile phases in column and gas-liquid chromatography
Describe the process of column chromatography
Suggest a material suitable for a solid phase and explain the adsorption and desorption processes
Explain why column chromatography can be used to purify a substance
Explain what a retention time is and how to measure these in GLC
State some conditions which must be standardised to compare retention times with a database to identify a substance
Explain retention times in terms of solubility in mobile and stationary
phases (more soluble in stationary phase means longer retention)
Explain how GLC separates the components of a mixture
Calculate a solutes relative proportion from GLC ( height x ½base)
Understand how GLC-MS are used in conjunction, giving examples
Identify atoms which possess the property of spin, limited to H and C
Describe how spin can be detected to create NMR spectra
Know that Tetramethylsilane (TMS), Si(CH
3
)
4
is used as a standard in
NMR
Give reasons why TMS is suitable for use as a standard in NMR
Identify the number of non-equivalent C and H environments in molecules or from given NMR spectra
Identify the simplest ratio of C and H in non-equivalent environments in molecules or from given NMR spectra
Understand the purpose of an integration trace
Know that electronegative species cause de-shielding and increased chemical shift
Interpret chemical shift data for C and H using the data sheet
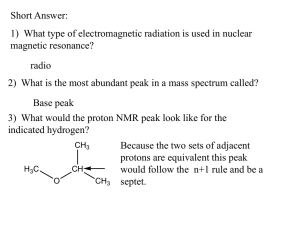
Know that spin-spin coupling of non-equivalent adjacent H causes peak splitting
Use the n+1 rule to determine peak splitting patterns in molecules
Identify singlets, doublets, triplets, quadruplets and multiplets
Know that homotopic groups will not cause splitting of each other e.g. in ethane, CH
3
CH
3
Use NMR spectra to determine the structure of a molecule, or sketch an NMR spectrum for a given molecule
Explain why water or ethanol are not suitable solvents for the sample being analysed in proton NMR
Know the difference between hydrogen, H 1 and deuterium, D 2
Explain why deutrated solvents e.g. CCl
4
or CDCl
3
are used in proton NMR
Optional: Explain how deuterium oxide, D
2
O, can be used to identify
–OH and –NH hydrogens (labile protons) by proton exchange
Optional: Represent the above proton exchange with symbol equations