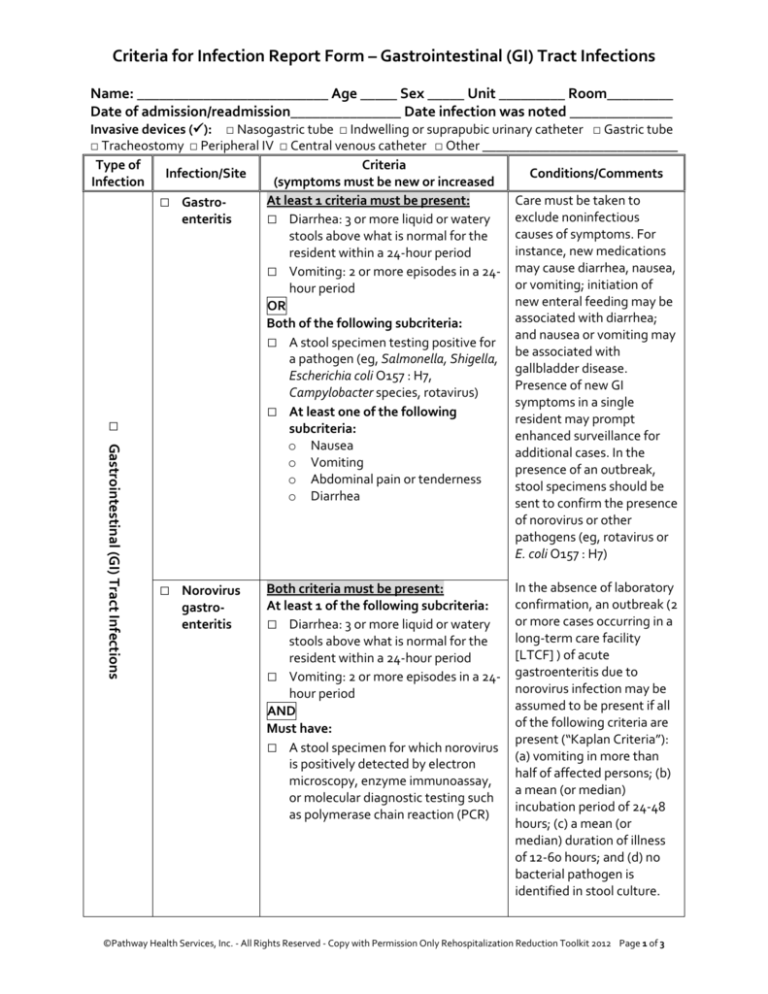
Criteria for Infection Report Form – Gastrointestinal (GI) Tract Infections
Name: __________________________ Age _____ Sex _____ Unit _________ Room_________
Date of admission/readmission_______________ Date infection was noted ______________
□ Gastrointestinal (GI) Tract Infections
Invasive devices (): □ Nasogastric tube □ Indwelling or suprapubic urinary catheter □ Gastric tube
□ Tracheostomy □ Peripheral IV □ Central venous catheter □ Other _____________________________
Type of
Criteria
Infection/Site
Conditions/Comments
Infection
(symptoms must be new or increased
At least 1 criteria must be present:
Care must be taken to
□ Gastroexclude noninfectious
enteritis
□ Diarrhea: 3 or more liquid or watery
causes of symptoms. For
stools above what is normal for the
instance, new medications
resident within a 24-hour period
□ Vomiting: 2 or more episodes in a 24- may cause diarrhea, nausea,
or vomiting; initiation of
hour period
new enteral feeding may be
OR
associated with diarrhea;
Both of the following subcriteria:
and nausea or vomiting may
□ A stool specimen testing positive for
be associated with
a pathogen (eg, Salmonella, Shigella,
gallbladder disease.
Escherichia coli O157 : H7,
Presence of new GI
Campylobacter species, rotavirus)
symptoms in a single
□ At least one of the following
resident may prompt
subcriteria:
enhanced surveillance for
o Nausea
additional cases. In the
o Vomiting
presence of an outbreak,
o Abdominal pain or tenderness
stool specimens should be
o Diarrhea
sent to confirm the presence
of norovirus or other
pathogens (eg, rotavirus or
E. coli O157 : H7)
□ Norovirus
gastroenteritis
Both criteria must be present:
At least 1 of the following subcriteria:
□ Diarrhea: 3 or more liquid or watery
stools above what is normal for the
resident within a 24-hour period
□ Vomiting: 2 or more episodes in a 24hour period
AND
Must have:
□ A stool specimen for which norovirus
is positively detected by electron
microscopy, enzyme immunoassay,
or molecular diagnostic testing such
as polymerase chain reaction (PCR)
In the absence of laboratory
confirmation, an outbreak (2
or more cases occurring in a
long-term care facility
[LTCF] ) of acute
gastroenteritis due to
norovirus infection may be
assumed to be present if all
of the following criteria are
present (“Kaplan Criteria”):
(a) vomiting in more than
half of affected persons; (b)
a mean (or median)
incubation period of 24-48
hours; (c) a mean (or
median) duration of illness
of 12-60 hours; and (d) no
bacterial pathogen is
identified in stool culture.
©Pathway Health Services, Inc. - All Rights Reserved - Copy with Permission Only Rehospitalization Reduction Toolkit 2012 Page 1 of 3
Criteria for Infection Report Form – Gastrointestinal (GI) Tract Infections
Type of
Infection
Infection/Site
□ Clostridium
difficile
infection
□ Gastrointestinal (GI) Tract Infections
Criteria
(symptoms must be new or increased
Both criteria must be present:
At least 1 of the following subcriteria:
□ Diarrhea: 3 or more liquid or watery
stools above what is normal for the
resident within a 24-hour period
□ Presence of toxic megacolon
(abnormal dilatation of the large
bowel, documented radiologically)
AND
At least 1 of the following subcriteria:
□ A stool sample yields a positive
laboratory test result for C. difficile
toxin A or B, or a toxin producing C.
difficile organism is identified from a
stool sample culture or by a
molecular diagnostic test such as
PCR
□ Pseudomembranous colitis is
identified during endoscopic
examination or surgery or in
histopathologic examination of a
biopsy specimen
Conditions/Comments
A “primary episode” of C.
difficile infection is defined
as one that has occurred
without any previous history
of C. difficile infection or
that has occurred >8weeks
after the onset of a previous
episode of C. difficile
infection. A “recurrent
episode” of C. difficile
infection is defined as an
episode of C. difficile
infection that occurs 8
weeks or sooner after the
onset of a previous episode,
provided that the symptoms
from the earlier (previous)
episode have resolved.
Individuals previously
infected with C. difficile may
continue to remain
colonized even after
symptoms resolve. In the
setting of an outbreak of GI
infection, individuals could
have positive test results for
presence of C. difficile toxin
because of ongoing
colonization and also be
coinfected with another
pathogen. It is important
that other surveillance
criteria be used to
differentiate infections in
this situation.
©Pathway Health Services, Inc. - All Rights Reserved - Copy with Permission Only Rehospitalization Reduction Toolkit 2012 Page 2 of 3
Criteria for Infection Report Form – Gastrointestinal (GI) Tract Infections
1. Was resident hospitalized due to this infection?
□ Yes
□ No
2. Culture results (if any):
DATE:
SITE:
ORGANISM(S):
COMMENTS:
DATE:
SITE:
ORGANISM(S):
COMMENTS:
DATE:
SITE:
ORGANISM(S):
COMMENTS:
3. Outcome; at end of infection, the resident was:
□ The same or better than before infection
□ More dependent that before infection
□ Transferred to another facility
□ Expired/deceased
4. Does resident have a multi-drug resistant organism on culture (eg, MRSA, VRE)?
□ Yes
□ No
5. If yes, type:
□ MRSA
□ VRE
□ C-Diff
□ Other:___________________________________
6. If culture positive for multi-drug resistant organism, do they meet criteria for infection
at the site of positive culture?
□ Yes
□ No (If no, resident is likely only colonized and not infected. Isolation or contact
precautions may be necessary.)
Comments:_____________________________________________________________________
_______________________________________________________________________________
_______________________________________________________________________________
_______________________________________________________________________________
Completed by:_________________________________ Title:____________ Date:___________
Source: Infection Control and Hospital Epidemiology 2012;33(10):965-977
©Pathway Health Services, Inc. - All Rights Reserved - Copy with Permission Only Rehospitalization Reduction Toolkit 2012 Page 3 of 3