File - MHS
advertisement

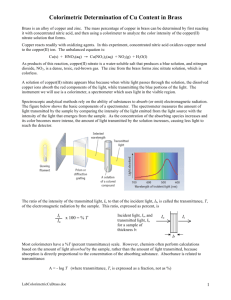
AP Chemistry: How Much Copper is Present? The primary objective of this experiment is to determine the copper concentration of an unknown brass sample. Molar concentrations of colored solutions (in this case copper nitrate) can be determined using a Colorimeter. Light is absorbed by colored solutions; concentrations there for can be determined using by passing a wavelength of light that will be absorbed through the solution. A higher concentration of the colored solution absorbs more light (and transmits less) than a solution of lower concentration. You will prepare five copper nitrate solutions of known concentration (standard solutions). Each is transferred to a small, rectangular cuvette that is placed into the Colorimeter or Spectrometer. The amount of light that penetrates the solution and strikes the photocell is used to compute the absorbance of each solution. When a graph of absorbance vs. concentration is plotted for the standard solutions, a direct relationship should result, as shown in Figure 1. The direct relationship between absorbance and concentration for a solution is known as Beer’s law: A=bc Figure 1 Where A is absorbance (no units, since A = log10 P0 / P ) is the molar absorbtivity with units of L mol-1 cm-1 b is the path length of the sample (i.e. the width of the cuvette, generally 1 cm) c is the concentration of the compound in mol/L In this experiment, students will measure the absorbance of known dilutions of copper nitrate to develop a calibration curve and determine b (the slope of the line). The concentration of the unknown can be found using the slope of the Beer’s law curve. Pre-Lab Questions 1. Read the procedure and create table to capture the data for this lab. 2. If 10.000 g of copper is measured out, how many moles of copper will be in the solution? 3. If the solution is diluted to 500.0 mL, what will be the molarity of the solution? 4. Based on this molarity, write a set of procedures to describe how you are your partner plan on making four, 20 mL solutions of the following concentrations. 0.300 M, 0.150 M and 0.0750 M and 0.0375 M. You may only use 20 ml of the original stock solution. Show any necessary calculations in your lab notebook. Making the Solution & Beer’s law 1. A solution of copper nitrate has been made for your class by dissolving approximately 10 grams of copper metal and diluting it to 500.0 ml in a volumetric flask. Record the mass of copper used in your period. 2. A sample of brass was also with nitric acid and diluted to 500.0 ml. Record all observations of this solution including the mass of the brass sample. 3. Calculate how to make 5 known dilutions of copper nitrate. Have your teacher approve your procedures for making your solutions. Be sure to record the ACTUAL mass of Cu used for your class and the ACTUAL volume of the stock solution used. Report these in your lab book. 4. Once approved, carry out your dilutions. Label each solution. 5. Use the colorimeter to find the absorbance of each solution. Record these values. 6. Test the brass solution and find its absorbance (an absorbance greater than 1 is not allowed. You may need to dilute your solution.) Results/Calculations: 1. Calculate the actual molarity of each of your dilutions. Volume of stock solution, actual molarity and measured absorbance should be included in a clearly labeled data table. 2. Graph your absorbance values on your calculator or a graphing program and find the equation of the line. Include the graph and the equation in your lab report. FOLLOW the lab manual graphing guidelines. 3. Using the equation of the line you found for the copper solutions, find the concentration of the copper in the brass solution. Show all work. 4. Using the concentration of the brass, find the percent of the copper in the shell casing. Post Lab Questions 1. A student is instructed to determine the concentration of a NiSO4 solution using a spectrophotometer or calorimeter. The student prepares these standards: 0.08 M, 0.16 M, 0.24 M, 0.32 M and 0.40 M NiSO4. The student then measures the absorption spectrum of the NiSO4 solution to determine an appropriate wavelength for the analysis. The following graph represents the data. a. Identify the optimum wavelength for the analysis. b. The student measures the absorbance of the 0.08 M, 0.16 M, 0.24 M, 0.32 M and 0.40 M solutions. The data is plotted below. Absorbance of the unknown solution is 0.750. What is the concentration of the solution? 2. A student handles the cuvette during the experiment and leaves fingerprints on the surface. How will this affect the calculated concentration of the unknown? Explain your answer (you may use equations). 3. Why is this method of determining the concentration of nickel (II) sulfate solution appropriate, whereas using the same method for measuring the concentration of a sodium chloride solution would not be appropriate