Applied Notes: Unit 6A—Chemical Reactions
advertisement
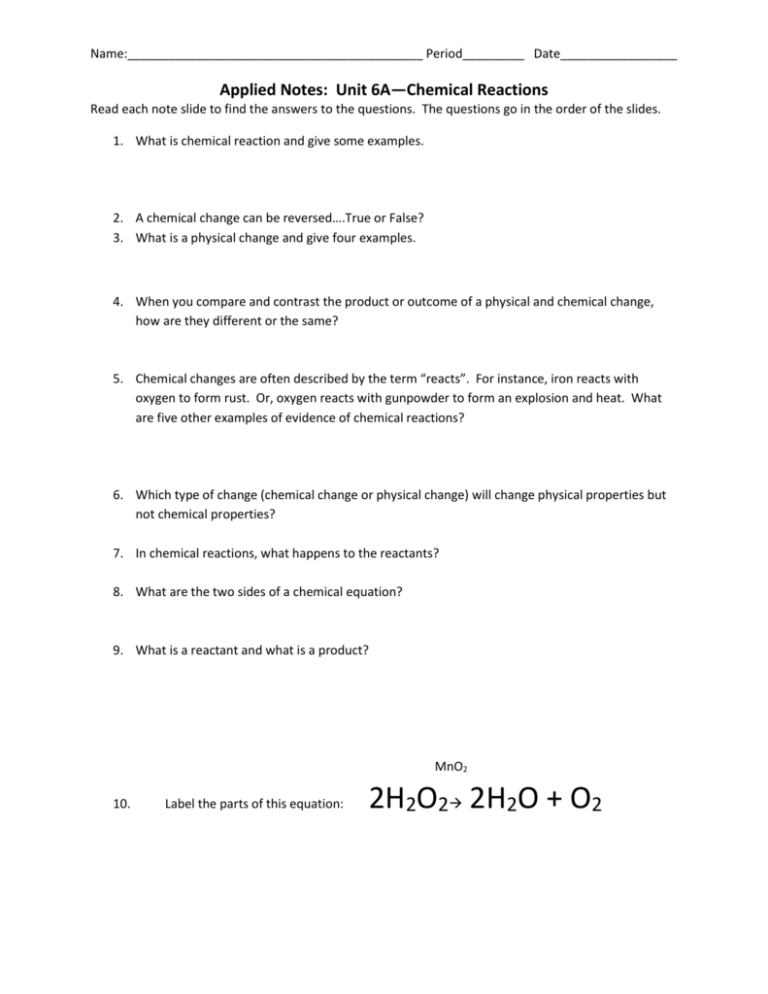
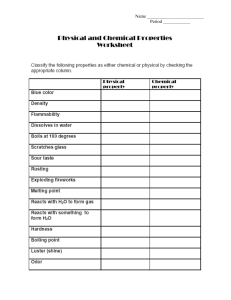
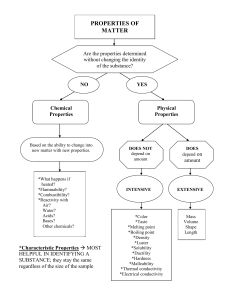
Name:___________________________________________ Period_________ Date_________________ Applied Notes: Unit 6A—Chemical Reactions Read each note slide to find the answers to the questions. The questions go in the order of the slides. 1. What is chemical reaction and give some examples. 2. A chemical change can be reversed….True or False? 3. What is a physical change and give four examples. 4. When you compare and contrast the product or outcome of a physical and chemical change, how are they different or the same? 5. Chemical changes are often described by the term “reacts”. For instance, iron reacts with oxygen to form rust. Or, oxygen reacts with gunpowder to form an explosion and heat. What are five other examples of evidence of chemical reactions? 6. Which type of change (chemical change or physical change) will change physical properties but not chemical properties? 7. In chemical reactions, what happens to the reactants? 8. What are the two sides of a chemical equation? 9. What is a reactant and what is a product? MnO2 10. Label the parts of this equation: 2H2O2 2H2O + O2 11. What is a catalyst? 12. Energy is stored in the metallic, covalent and ionic bonds between chemicals. What happens to the energy when the bonds are made? _______________________________ What happens to the energy when the bonds are broken?____________________________________________ 13. What is it called when energy leaves or exits a reaction?_______________________________ 14. What is it called when energy is taken in or enters a reaction?__________________________ 15. What is the symbol we use to show that heat is added to a reaction? 16. Show how we abbreviate the following terms in a chemical equation: Liquid-________ Solid-_______ 17. What does aqueous mean? Aqueous-_______ Gas-_______