Word - My eCoach
advertisement
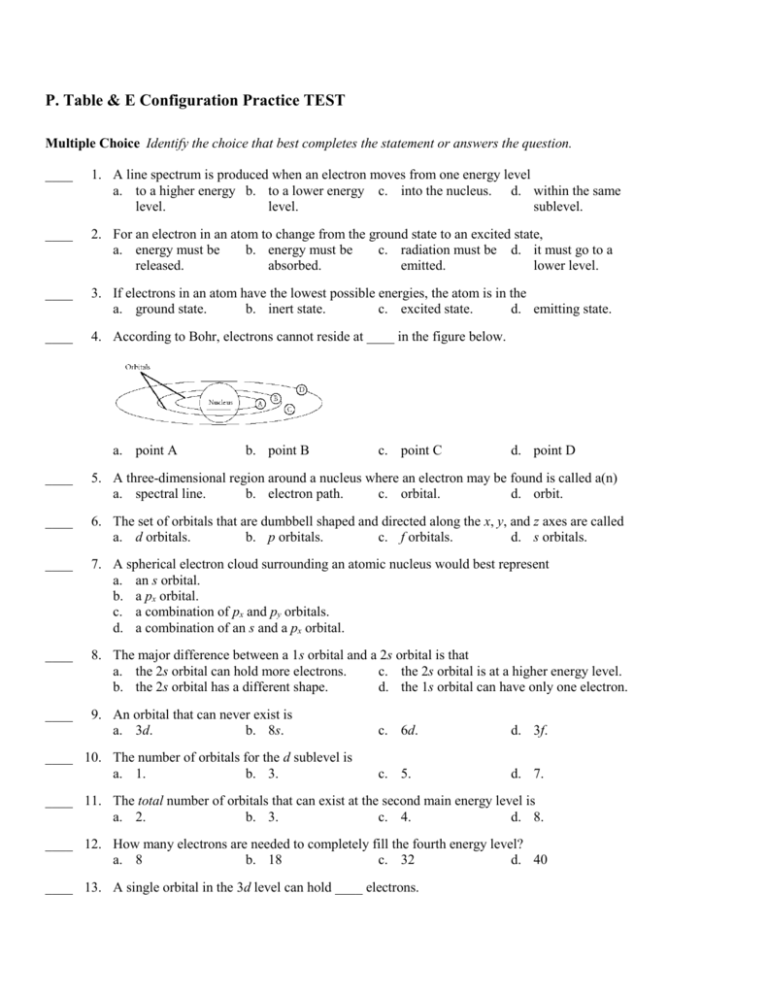
P. Table & E Configuration Practice TEST Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. A line spectrum is produced when an electron moves from one energy level a. to a higher energy b. to a lower energy c. into the nucleus. d. within the same level. level. sublevel. ____ 2. For an electron in an atom to change from the ground state to an excited state, a. energy must be b. energy must be c. radiation must be d. it must go to a released. absorbed. emitted. lower level. ____ 3. If electrons in an atom have the lowest possible energies, the atom is in the a. ground state. b. inert state. c. excited state. d. emitting state. ____ 4. According to Bohr, electrons cannot reside at ____ in the figure below. a. point A b. point B c. point C d. point D ____ 5. A three-dimensional region around a nucleus where an electron may be found is called a(n) a. spectral line. b. electron path. c. orbital. d. orbit. ____ 6. The set of orbitals that are dumbbell shaped and directed along the x, y, and z axes are called a. d orbitals. b. p orbitals. c. f orbitals. d. s orbitals. ____ 7. A spherical electron cloud surrounding an atomic nucleus would best represent a. an s orbital. b. a px orbital. c. a combination of px and py orbitals. d. a combination of an s and a px orbital. ____ 8. The major difference between a 1s orbital and a 2s orbital is that a. the 2s orbital can hold more electrons. c. the 2s orbital is at a higher energy level. b. the 2s orbital has a different shape. d. the 1s orbital can have only one electron. ____ 9. An orbital that can never exist is a. 3d. b. 8s. ____ 10. The number of orbitals for the d sublevel is a. 1. b. 3. c. 6d. d. 3f. c. 5. d. 7. ____ 11. The total number of orbitals that can exist at the second main energy level is a. 2. b. 3. c. 4. d. 8. ____ 12. How many electrons are needed to completely fill the fourth energy level? a. 8 b. 18 c. 32 d. 40 ____ 13. A single orbital in the 3d level can hold ____ electrons. a. 10 b. 2 c. 3 ____ 14. The atomic sublevel with the next highest energy after 4p is a. 4d. b. 4f. c. 5p. d. 6 d. 5s. ____ 15. In the electron configuration for scandium (atomic number 21), what is the notation for the three highestenergy electrons? a. 3d1 4s2 b. 4s3 c. 3d3 d. 4s2 4p1 ____ 16. What is the electron configuration for nitrogen, atomic number 7? a. 1s2 2s2 2p3 b. 1s2 2s3 2p2 c. 1s2 2s3 2p1 d. 1s2 2s2 2p2 3s1 ____ 17. The number of electrons in the highest energy level of the argon atom (atomic number 18) is a. 10. b. 2. c. 6. d. 8. ____ 18. The idea of arranging the elements in the periodic table according to their chemical and physical properties is attributed to a. Mendeleev. b. Moseley. c. Bohr. d. Ramsay. ____ 19. Mendeleev noticed that properties of elements usually repeated at regular intervals when the elements were arranged in order of increasing a. atomic number. b. density. c. reactivity. d. atomic mass. ____ 20. Moseley's work led to the realization that elements with similar properties occurred at regular intervals when the elements were arranged in order of increasing a. atomic mass. b. density. c. radioactivity. d. atomic number. ____ 21. Argon, krypton, and xenon are a. alkaline earth b. noble gases. metals. c. actinides. d. lanthanides. ____ 22. Elements in a group or column in the periodic table can be expected to have similar a. atomic masses. b. atomic numbers. c. # of neutrons. d. properties. ____ 23. The atomic # of lithium, the first element in Group 1, is 3. The atomic#of the 2nd element in this group is a. 4. b. 10. c. 11. d. 18. ____ 24. To what group on the periodic table do chlorine & fluorine belong? a. alkaline-earth b. transition c. halogens metals elements d. actinides ____ 25. A horizontal row of blocks in the periodic table is called a(n) a. group. b. period. c. family. d. octet. ____ 26. Refer to the figure above. Potassium and bromine belong to a. Period 4. b. Group 4. c. Period 1. d. Group 1. ____ 27. Identify the sublevels in a period that contains 32 elements. a. s, f b. s, p c. s, p, d d. s, p, d, f ____ 28. Elements to the right side of the periodic table (p-block elements) have properties most associated with a. gases. b. nonmetals. c. metals. d. metalloids. ____ 29. Elements in which the d-sublevel is being filled have the properties of a. metals. b. nonmetals. c. metalloids. d. gases. ____ 30. The group of 14 f block elements in the sixth period is the a. actinides. b. lanthanides. c. transition elements. d. metalloids. ____ 31. Within the p-block elements, the elements at the top of the table, compared with those at the bottom, a. have larger radii. c. have lower ionization energies. b. are more metallic. d. are less metallic. ____ 32. The electron configurations of the noble gases from neon to radon in the periodic table make these elements part of the a. f block. b. d block. c. s block. d. p block. ____ 33. To which block do the actinide elements belong? a. d block b. s block c. f block d. p block ____ 34. The element that has the greatest electronegativity is a. oxygen. b. sodium. c. chlorine. d. fluorine. ____ 35. A negative ion is known as a(n) a. ionic radius. b. valence electron. d. anion. c. cation. Short Answer 36. a. Identify the number of valence electrons b. How many unpaired electrons are there? c. Write the electron configuration for the arrow diagram below. 37. The electron configuration for nitrogen is 1s2 2s2 2p3. What does the 3 in 2p3 mean? 38. In terms of the periodic law, explain which two of these elements are most similar: sodium (element 11), phosphorus (element 15), and sulfur (element 16). 39. Write the electron configuration for nitrogen, atomic number 7. 40. Draw the orbital diagram for argon. P. Table & E Configuration Practice TEST Answer Section MULTIPLE CHOICE 1. ANS: OBJ: 2. ANS: OBJ: 3. ANS: OBJ: 4. ANS: OBJ: 5. ANS: OBJ: 6. ANS: OBJ: 7. ANS: OBJ: 8. ANS: OBJ: 9. ANS: OBJ: 10. ANS: OBJ: 11. ANS: OBJ: 12. ANS: OBJ: 13. ANS: OBJ: 14. ANS: OBJ: 15. ANS: OBJ: 16. ANS: OBJ: 17. ANS: OBJ: 18. ANS: OBJ: 19. ANS: OBJ: 20. ANS: OBJ: 21. ANS: OBJ: 22. ANS: B 3 B 4 A 4 C 4 C 3 B 5 A 5 C 5 D 5 C 5 C 5 C 1 B 1 D 2 A 3 A 3 D 3 A 1 D 1 D 1 B 2 D PTS: 1 DIF: II REF: 1 PTS: 1 DIF: II REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: III REF: 1 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: 1 SC.A.2.4.6 1 SC.A.1.4.1 1 SC.A.1.4.1 1 SC.A.1.4.1 1 SC.A.1.4.1 1 SC.A.1.4.1 1 SC.A.1.4.1 1 SC.A.2.4.5 1 SC.A.2.4.5 1 DIF: I REF: 2 DIF: I REF: 2 DIF: II REF: 2 DIF: II REF: 2 DIF: II REF: 2 DIF: II REF: 2 DIF: II REF: 2 DIF: II REF: 3 DIF: II REF: 3 DIF: II REF: 3 PTS: STA: PTS: STA: PTS: STA: PTS: 1 SC.A.2.4.5 1 SC.A.2.4.5 1 SC.A.2.4.5 1 DIF: III REF: 3 DIF: II REF: 3 DIF: II REF: 3 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 STA: SC.A.2.4.5 PTS: 1 DIF: I REF: 1 DIF: I REF: 1 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: C C B A D B A B D D C D D PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 1 1 1 1 1 DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: DIF: II I I I II I I I I I I II I REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: REF: 1 1 1 1 2 2 2 2 2 2 2 3 3 SHORT ANSWER 36. ANS: a. There are 6 valence electrons in the atom shown. b. There are 2 unpaired electrons in the 2p sublevel of the atom shown. c. 1s2 2s2 2p4 37. ANS: The 3 in 2p3 indicates that three electrons are in the p orbitals of the second energy level. 38. ANS: Their locations in the periodic table indicate that phosphorus and sulfur are nonmetals and sodium is a metal. Nonmetals are a group with characteristic properties, so phosphorus and sulfur are the most similar elements of the three. 39. ANS: 1s2 2s2 2p3 40. ANS: Please note: i could not insert arrows, so the “^” represents an upward facing arrow and the “v” represents a downward facing arrow. 3p ^v ^v ^v 3s ^v 2p ^v ^v ^v 2s ^v 1s ^v









