Chapter 12 – Stoichiometry Note Taking Guide: Episode 801
advertisement
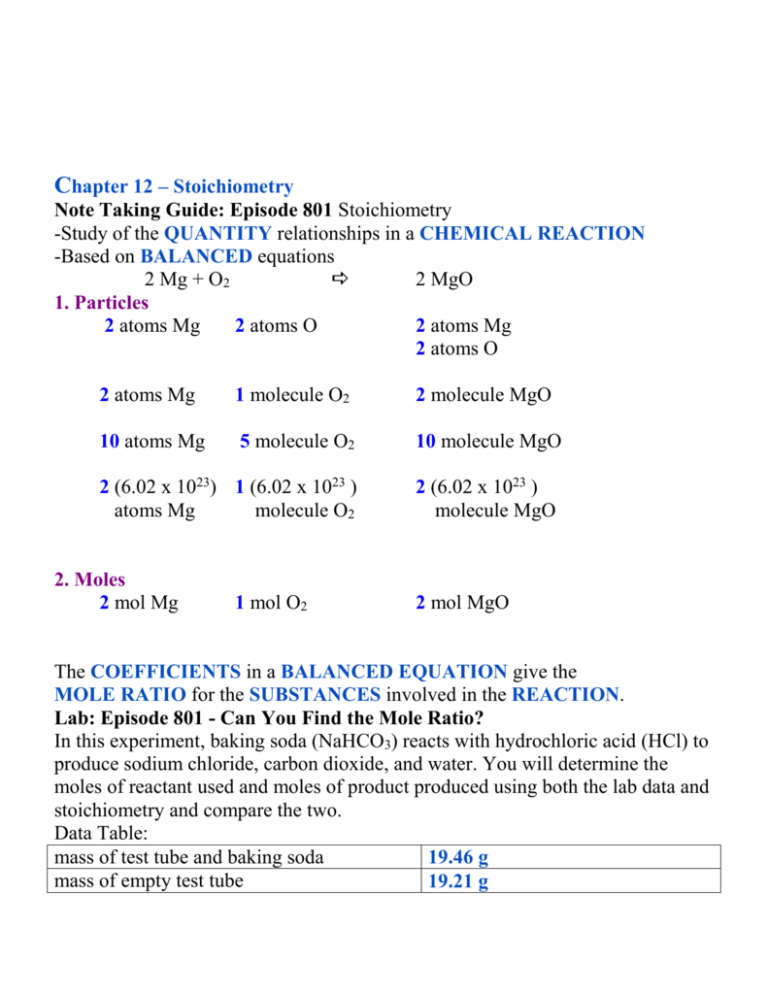
Chapter 12 – Stoichiometry Note Taking Guide: Episode 801 Stoichiometry -Study of the QUANTITY relationships in a CHEMICAL REACTION -Based on BALANCED equations 2 Mg + O2 2 MgO 1. Particles 2 atoms Mg 2 atoms O 2 atoms Mg 2 atoms O 2 atoms Mg 1 molecule O2 2 molecule MgO 10 atoms Mg 5 molecule O2 10 molecule MgO 2 (6.02 x 1023) 1 (6.02 x 1023 ) atoms Mg molecule O2 2 (6.02 x 1023 ) molecule MgO 2. Moles 2 mol Mg 1 mol O2 2 mol MgO The COEFFICIENTS in a BALANCED EQUATION give the MOLE RATIO for the SUBSTANCES involved in the REACTION. Lab: Episode 801 - Can You Find the Mole Ratio? In this experiment, baking soda (NaHCO3) reacts with hydrochloric acid (HCl) to produce sodium chloride, carbon dioxide, and water. You will determine the moles of reactant used and moles of product produced using both the lab data and stoichiometry and compare the two. Data Table: mass of test tube and baking soda 19.46 g mass of empty test tube 19.21 g mass of baking soda 0.25 g mass of test tube and sodium chloride 19.39 g mass of empty test tube 19.21 g mass of sodium chloride 0.18 g Conclusion Questions: 1. Calculate the number of moles of baking soda used in the lab. ? mol NaHCO3 = 0.25 g NaHCO3|1 mol NaHCO3 = 0.0030 mol NaHCO3 |84.0 g NaHCO3 23.0 + 1.01 + 12.0 + 3(16.0) = 84.0 2. Calculate the number of moles of sodium chloride produced in the lab. ? mol NaCl = 0.18 g NaCl|1 mol NaCl = 0.0031 mol NaCl | 58.5 g NaCl 3. What is the experimental mole ratio of baking soda (NaHCO3) to sodium chloride (NaCl)?0.0030: 0.0031 or 1:1 4. Write a balanced equation for the reaction that took place in the experiment. NaHCO3 + HCl NaCl + H2O + CO2 (no coefficients needed) 5. According to the balanced equation, what is the theoretical mole ratio of baking soda to sodium chloride? 1:1 6. Was the experimental mole ratio exactly the same as the theoretical mole ratio? If not, give some possible reasons. Although they should be the same, the experimental mole ratio may be slightly different than the theoretical mole ratio due to experimental error such as not allowing the test tube to be completely cooled when massing at the end or allowing some of the liquid to splatter out of the test tube when heating. Maybe the NaCl was not perfectly dried. Ex. Problem: When elemental aluminum reacts with elemental iodine, aluminum iodide is produced. The balanced equation is: 2Al + 3I2 2AlI3 mole ratios: 2 Al: 3 I2 2 Al: 2 AlI3 3 I2: 2 AlI3 If you start with 4 moles of Al, how many moles of AlI3 will be produced? 4 mol Al | 2 mol AlI3 = 4 mol AlI3 2 mol Al Problem Set One 2N2H4 + N2O4 3N2 + 4H2O BE SURE TO BALANCE THE EQUATION FIRST!! ? moles N2O4 = 2.72 moles N2H4 ? moles N2 = 2.72 moles N2H4 2.72 moles N2H4 | 1 mole N2O4 = 1.36 mol N2O4 2 moleN2H4 2.72 moles N2H4 | 3 mole N2 | 2 moleN2H4 = 4.08 mol N2 How many moles of water will be produced when 8.0 grams of hydrogen gas react with the oxygen in the air? Balanced equation : 2H2 + O2 2H2O (Hint: To “make the switch” between different substances in a reaction, use the MOLE ratio from the BALANCED equation.) ? moles H2O = 8.0 g H2 8.0 g H2 | 1 mole H2 | 2 mole H2O = 3.96 mole H2O | 2.02 g H2 | 2 mol H2 Problem Set Two In photosynthesis, carbon dioxide and water react to form glucose, C6H12O6 and oxygen gas. BE SURE TO BALANCE THE EQUATION FIRST!! 15.6 g ? mol 6 CO2 + 6 H2O C6H12O6 + 6 O2 If 15.6 grams of carbon dioxide react, how many moles of glucose will be produced? 15.6 g CO2 = ? moles C6H12O6 15.6 g CO2 | 1 mol CO2 | 1 mol C6H12O6 = 0.0591 mol C6H12O6 | 44.0 g CO2 | 6 mol CO2 (Molar mass) How many grams of carbon dioxide must react to produce 0.25 mole of glucose? ? g CO2= 0.25 moles C6H12O6 0.25 moles C6H12O6| 6 mol CO2 | 44.0 g CO2 = 66.0 g CO2 | 1 mol C6H12O6 | 1 mol CO2 The Chemistry Quiz CR1. B. molar mass CR2 .C COEFFICIENTS 1. B. QUANTITY 2. B. 2 mol H2 3. B. Mole Ratio 4. B. 16.6 moles 5. A. 298.8 g Note Taking Guide: Episode 802 Problem: When nitrogen and hydrogen react they form ammonia gas, NH3. If 56.0 g of nitrogen are used up in the reaction how many grams of ammonia are produced? 1) Write the balanced equation N2 + 3H2 2NH3 2) Write it in question form. ? g NH3 = 56.0 g N2 3) Convert mass to moles using molar mass 4) Use the mole ratio to move from moles of N2 to moles of NH3 5) Use the molar mass of NH3 to find grams of NH3 56.0 g N2 | 1 mol N2 | 2 mol NH3 | 17.0 g NH3 = 68.0 g NH3 | 28.0 g N2| 1 mol N2 | 1 mol NH3 Ex. Problems: Sodium metal reacts with oxygen gas to produce solid sodium oxide. How many grams of sodium must react to produce 42.0 grams of sodium oxide? WRITE THE BALANCED EQUATION FIRST!!!! ?g 42.0 g 4Na + O2 2Na2O ? g Na = 42.0 g Na2O 42.0 g Na2O| 1 mol Na2O | 4 mol Na | 23.0 g Na = 31.2 g Na | 62.0 g Na2O | 2 mol Na2O |1 mol Na When 12.0 g of hydrogen reacts with oxygen, how many grams of water are produced? 12.0 g ?g 2H2 + O2 2H2O 12.0 g H2 | 1 mol H2 | 2 mol H2O | 18.0 g H2O = 107 g H2O | 2.02 g H2 | 2 mol H2 |1 mol H2O Actual Yield: amount of PRODUCT produced when the REACTION is performed in a LAB. Theoretical Yield: amount of PRODUCT expected to be PRODUCED based on the BALANCED EQUATION and the amount of REACTANTS Percent Yield (ACTUAL yield / THEORETICAL yield) x 100% Percent Yield in Lab actual yield of CO2 = ____________ (from lab data) Calculate theoretical yield from balanced equation: Mass of sodium bicarbonate MASS BEFORE THE REACTION MASS AFTER THE REACTION Mass of CO2 = 5.98 g - 5.85 g = 0.13 g 0.23 g 5.98 g 5.85 g = ACTUAL yield Theoretical yield 0.23 g ?g NaHCO3 + HCl NaCl + CO2 + H2O ?g CO2 = 0.23 g NaHCO3 0.23 g NaHCO3 | 1 mol NaHCO3 | 1 mol CO2 | 44.0 g CO2 = 0.12 g CO2 |84.0 g NaHCO3 | 1 mol NaHCO3 | 1 mol CO2 % yield = 0.13 g/ 0.12 x 100% = 108% What is the % yield of carbon dioxide when 5.99 grams of propane are burned and 12.052 grams of carbon dioxide are collected? ? theoretical 5.99g 12.052 g actual C3H8 + 5 O2 3CO2 + 4 H2O Theoretical 5.99 g C3H8 | 1 mol C3H8 | 3 mol CO2 | 44.0 g CO2 = 17.9 g CO2 | 44.1 g C3H8 | 1 mol C3H8 | 1 mol CO2 %yield = 12.052 g / 17.9 g x 100 = 67.3 % The Chemistry Quiz CR1. CR2. 1. 2. 3. 4. 5. Interpreting Chemical Equations ATOMS and MASS are conserved in a balanced equation. Mass-Particle Calculations EX 4: How many molecules of O2 are produced when 29.2 g of H2O is decomposed? 29.2 g ? molecules 2 H2O(g) 1 O2(g) + 2 H2(g) 1. Convert mass to moles. 2. Use the mole ratio to go from moles of H2O to moles of O2. 3. Convert moles of O2 to molecules of O2 using 6.02 x 1023. 29.2 g H2O | 1 mol H2O | 1 mol O2 | 6.02 x 1023 molecules |18.0 g H2O| 2 mol H2O | 1 mol O2 23 = 4.88 x 10 molecules O2 Volume-Volume Calculations EX 5: Assuming STP, how many liter of O2 are needed to produce 19.8 L of SO3? ?L 19.8 L 2 SO2(g) + 1 O2(g) 2 SO3(g) 1. Convert liters of SO3(g) to moles using the molar volume, (22.4 L/mol) 2. Use the mole ratio to go from moles SO3 to moles of O2. 3. Use the molar volume to go from moles of O2 to liters. 19.8 L SO3 | 1 mol SO3 | 1 mol O2 | 22.4 L O2 = 9.90 L O2 | 22.4 L SO3 | 2 mol SO3 | 1 mol O2 OR The mole ratio also gives the volume ratio. Use the coefficients to find the volume ratio 19.8 L SO3 | 1 L O2 = 9.90 L O2 | 2 L SO3 http://www.dlt.ncssm.edu/TIGER/chem2.htm#stoich Note Taking Guide: Episode 803 Given that the density of oxygen is 1.439 g per liter, how many liters of oxygen gas can be produced if 15.0 g of mercury (II) oxide are heated to produce mercury and oxygen gas? ? L O2 = 15.0 g HgO BALANCE the equation 2HgO 2Mg + O2(g) Convert grams to MOLES. 15.0g HgO | 1 mol HgO | 216.6 g HgO Use the MOLE RATIO to switch from HgO to O2. 15.0 g HgO | 1 mol HgO | 1 mol O2| | 216.6 g HgO | 2 mol HgO Change MOLES to GRAMS using molar mass. 15.0 g HgO | 1 mole HgO | 1 mol O2 | 32.0 g O2 | 216.6 g HgO | 2 mol HgO | 1 mol O2 5. Use the DENSITY of O2 to change grams to LITERS 15.0 g HgO | 1 mole HgO | 1 mol O2 | 32.0 g O2 | 1 L O2 | 216.6 g HgO | 2 mol HgO | 1 mol O2 | 1.439 g O2 0.770 L O2 Stoichiometry Problems Guidelines BALANCE the equation 2. Convert to MOLES of GIVEN substance 3. Make the SWITCH using MOLE RATIO from the balanced equation. 4. Convert to UNIT needed in the problem. Problem Set One: = Given that the density of carbon dioxide is approximately 1.99 g/L, what volume of carbon dioxide will be produced if 85.5 g of pentane are burned? 85.5 g ?L C5H12 + 8O2 5CO2 + 6H2O 85.5 g C5H12 | 1 mol C5H12 | 5 mol CO2 | 44.0 g CO2 | 1 L CO2 = 131 L | 72.1 g C5H12 | 1 mol C5H12 | 1 mol CO2 | 1.99 g CO2 How many molecules of water will be produced if 26.3 g of pentane are burned? 26.3 g ? molecules C5H12 + 8O2 5CO2 + 6H2O 26.3 g C5H12 | 1 mol C5H12 | 6 mol H2O | 6.02 x 1023 molecules H2O | 72.1 g C5H12 | 1 mol C5H12 | 1 mol H2O 24 1.32 x 10 molecules WRITE THE BALANCED EQUATION FIRST!!!! Limiting Reactant REACTANT used up FIRST in a CHEMICAL reaction. Excess Reactant REACTANT that is not used up in a chemical REACTION Ex. Problem: When FeCl3 reacts with O2, Fe2O3 and Cl2 are produced. If 4.0 moles of FeCl3 and 4.0 moles of O2 are mixed, how many grams of Fe2O3 will be produced? 4.0 mol 4.0 mol ?g 4FeCl3 + 3 O2 2Fe2O3 + 6Cl2 4.0 mol FeCl3| 2 mol Fe2O3 | 159.6 g Fe2O3 = 320 g Fe2O3 | 4 mol FeCl3 | 1 mol Fe2O3 4.0 mol O2 | 2 mol Fe2O3 | 159.6 g Fe2O3 = 430 g Fe2O3 | 3 mol O2 | 1 mol Fe2O3 (Hint: Work two separate problems, using one reactant at a time.) (Hint: Answer will be the (smaller, larger) amount of product.) What is the limiting reactant? FeCl3 What is the excess reactant? O2 How many moles of O2 will be left after the reaction Do a mole-mole (Mass-mass) problem from the limiting reactant to the excess reactant 4.0 mol FeCl3 | 3 mol O2 = 3 mol O2 |4 mol FeCl3 Subtract the number of mole used from the number of moles of reactant O2. 4.0 mol O2 - 3 mol O2= 1 mol O2 Problem Set Two (Work on back.) BE SURE TO BALANCE THE EQUATION FIRST!! 0.18 g 0.11 g ?g Fe2O3 + 3CO 2Fe + 3CO2 Iron (III) oxide reacts with carbon monoxide to form molten iron and carbon dioxide. If 0.18 g of iron (III) oxide reacts with 0.11 g of carbon monoxide, how many grams of iron would be produced? What is the limiting reactant? Fe2O3What is the excess reactant? CO 0.18 g Fe2O3| 1 mol Fe2O3 | 2 mol Fe | 55.8 g Fe = 0.13 g Fe | 159.6 g Fe2O3 | 1 mol Fe2O3 | 1 mol Fe 0.11 g CO | 1 mol CO | 2 mol Fe | 55.8 g Fe = 0.15 g Fe | 28.0 g CO| 3 mol CO | 1 mol Fe The Chemistry QuizCR1. CR2. 1. 2.3. 4. 5. How many grams of CO will be left? 1. Find the number that was used. (Mass-mass from limiting to excess) 2. Subtract this from the initial amount. Limiting Excess 0.18 g Fe2O3| 1 mol Fe2O3 | 3 mol CO | 28.0 g CO = 0.095 g CO | 159.6 g Fe2O3 | 1 mol Fe2O3 | 1 mol CO 0.11g – 0.095g = 0.02 g CO left








