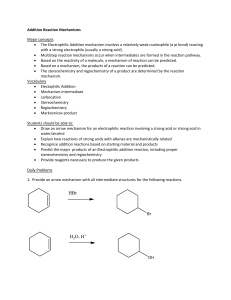
Experimental procedure for the synthesis of standard HCl
advertisement

Supporting Information Synthesis of decylphosphonic acid. Decylphosphonic acid was prepared by the Michaelis-Arbuzov reaction followed by an acid hydrolysis of the corresponding ester phosphonate. Decylbromide (5mL, 0.024 mol) and an excess of triethylphosphite (15mL, 0.087mol) were refluxed with a nitrogen stream bubbling into the solution at 150°C for 24h. The solution was cooled, and then 55mL of water was added in one portion. The two-phase mixture was stirred for 3h and extracted with chloroform (40ml). The organic phase, washed with saturated NaCl is then dried over MgSO4. The filtered solution was concentrated by rotary evaporation to give diethyldecylphosphonate, as yellow oil. Subsequently, this ester was hydrolysed by heating under reflux at 100°C with concentrated HCl (200ml) for 12h to yield decylphosphonic acid as a white precipitate upon cooling. DPA was purified by recrystallization with hexane. 1H NMR (400 MHz, CDCl3): 0.880 (t, 3H, C10), 1.258-1.362 (m, 14H, C3-C9), 1.607-1.736 (m, 4H, C1-C2), 11.255 (s, 2H, PO(OH)2); C NMR (100 MHz, CDCl3): 14.111 (s, CH3-CH2), 22.083 (d, 13 CH2CH2PO(OH)2, J2CP = 4.9 Hz), 22.685 (s, CH2), 26.153 (d, CH2PO(OH)2, J1CP = 144 Hz), 29.100-29.578 (4s, CH2), 30.596 (d, CH2CH2CH2PO(OH)2, J3CP = 17 Hz), 31.903 (s, CH2). Experimental procedure for the synthesis of standard HCl doped polyaniline PANI doped with HCl was synthesized by the slow addition, under stirring of 50 ml of a 1M HCl aqueous solution of ammonium peroxydisulfate (25 mmol) to 50 ml of a 1M HCl aqueous solution of aniline (20 mmol). 1 Table S1. Solubility (mg/ml) of DPA and HCl doped PANI in some common organic solvents Sample Solvent Chloroform Toluene n-heptane Ethanol ES-1,25-1 3.58 2.56 1.90 2.00 ES-1,25-1,5 3.89 2.88 1.82 2.21 ES-1,25-2,5 4.04 3.08 2.24 2.35 ES-1,25-5 4.57 3.46 2.62 2.55 PANI-HCl 0.72 0.66 0.56 0.50 2 10 µm Figure S1. Light micrograph of 3.79 mM DPA dispersed in water taken at room temperature. 3 (A) 48 h 24 h 600 min 580 min 560 min 540 min 480 min 0 min (B) 48 h 24 h 540 min 420 min 360 min 300 min 240 min 0 min Figure S2. UV-vis spectra of (a) ES-1.25-2.5 and (b) ES-1.25-1.5 dispersions recorded during polymerization and color changes of reaction mixture. 4 1,2 1 ES-1.25-1.5 0,8 Absorbance ES-1.25-2.5 ES-1.25-5 0,6 0,4 0,2 0 0 100 200 300 400 500 600 700 800 Polymerization time / min Figure S3. Changes in the absorbance of the polaron band at 680-750 nm with respect to polymerization time Viscosity(mPa.s) 12 8 4 0 1 1.5 2 2.5 3 3.5 4 4.5 5 [DcPA]/[An] FigureS4. Viscosity of the initial DPA micellar medium as a function of DPA/aniline molar ratio 5 1484 1578 815 1375 Y =5 Absorbance Y =2.5 Y=1.5 1040 Y=1 1700 1500 1300 1100 900 700 500 Wavenumber, cm-1 Figure S5. FTIR spectra of the dedoped PANIs in the region between 500 and 1700 cm -1 with Y= DPA/aniline molar ratio in the feed. 6 0,6 Fe concentration (ppm) 0,5 0,4 0,3 0,2 0,1 0 PVB PANI EB 2%/PVB PANI-HCl 2%/PVB ES-0.5-2.5/PVB Figure S6. Iron concentration after 10 days immersion test in 3.5% NaCl solution 7