Rules for Naming Compounds
advertisement

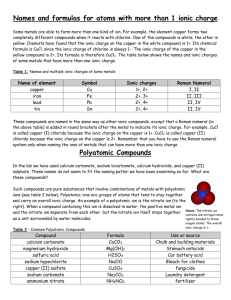
Rules for Naming Compounds Ionic Compounds (a compound of a metal and non-metal) Most of the Time: the name of the metal comes first (with the first letter capitalized) and the name of the non-metal comes second (without any capitalization) add the suffix "-ide" to the end of the name of the non-metal (ie. sulfur becomes sulfide) examples: KBr = Potassium bromide; MgS = Magnesium sulfide; CaI2 = Calcium iodide Exceptions to the Rule: if the metal is a transition metal, the rules change transition metals are metals that can form more than one kind of ion transition metals can create elements that have multiple ionic charges transition metals include elements with atomic number included in the ranges: 21-30, 39-48, 72-80, and 104-112 name these the same as any ionic compound, except after the metal, indicate the charge of the metal with Roman numerals in brackets examples: FeO = Iron (II) oxide because oxygen has a -2 charge and so would require a +2 charge from the iron; Fe2O3 = Iron (III) oxide because the 3 oxygen ions with a -2 charge (total of -6) and the 2 irons would need to have a -3 charge to balance out the oxygen Polyatomic Ions (an ion made up of two or more atoms that act as a group in bonding) Here are some common polyatomic ions: Polyatomic Name hydroxide chlorate chromate acetate nitrate nitrite hydrogen carbonate (bicarbonate) carbonate sulfate phosphate ammonium Formula OHClO3CrO42C2H3O2NO3NO2- Ionic Charge 112111- HCO3- 1- CO32SO42PO43NH4+ 2231+ rules for writing the formulas of polyatomic compounds is similar to ionic compounds, except place parentheses around the polyatomic ion naming polyatomic compounds: same rules as ionic compounds but don't add "-ide" to the polyatomic compound example: CuCO3 = Copper (II) carbonate because we know that the charge for carbonate is -2, our charge for copper must be +2 Molecular Compounds (a compound of two non-metals) the name of the binary molecular compound (one made of only two different elements) ends with the suffix "-ide" the name of the compound begins with the element that has the greater combining capacity use a prefix to specify the number of atoms of each element that is present in the molecule. The prefix "mono-" is only used for the second element and the "o" is dropped in the case of oxygen. prefixes include: mono (1); di (2); tri (3); tetra (4); penta (5); hexa (6) examples: CO = carbon monoxide; CO2 = carbon dioxide; P2S5 = Diphosphorous pentasulfide Diatomic Molecules (a molecule that contains two identical atoms) seven elements form compounds in this way: Hydrogen, Nitrogen, Oxygen, Flourine, Chlorine, Bromine, and Iodine examples: H2 = hydrogen gas; O2 = oxygen gas